Abstract
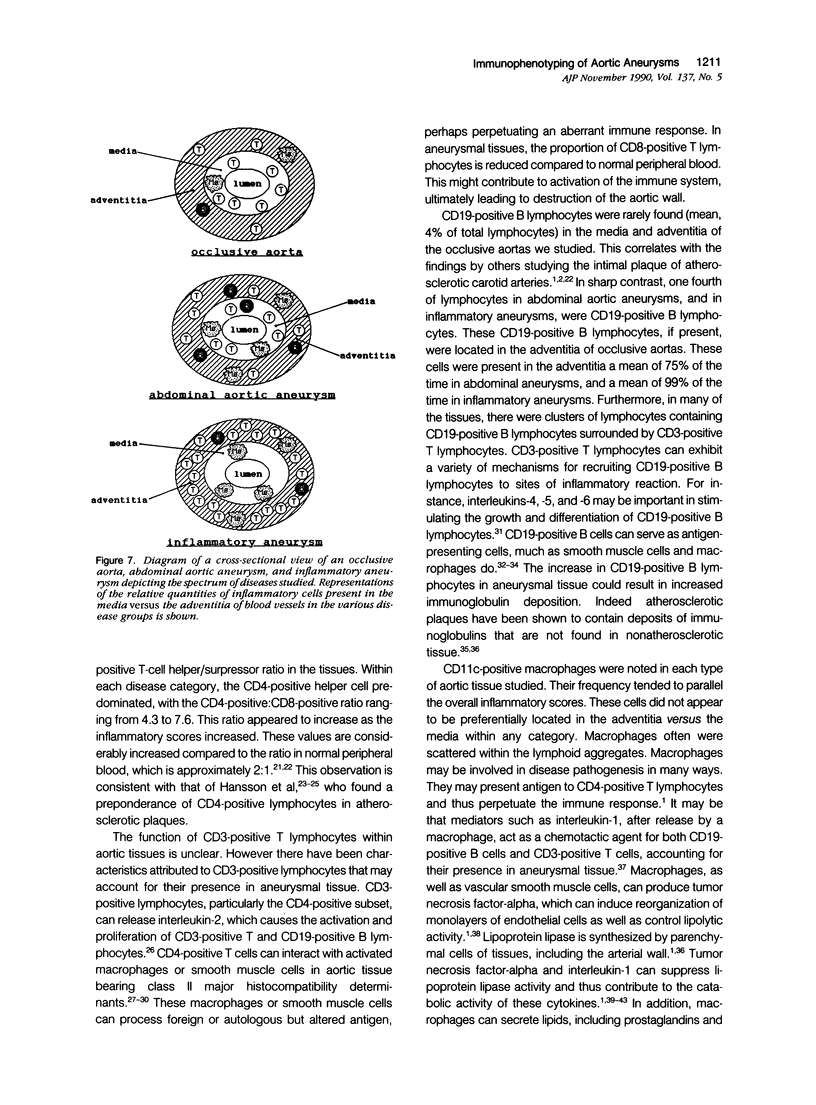
Cellular immunity may play a role in the pathogenesis of atherosclerosis. In this report the potential role of these cells in the formation of abdominal aortic aneurysms by immunohistochemistry was investigated. Aortic tissues from 32 patients were examined: 4 normal aortas, 6 aortas with occlusive atherosclerotic disease, 17 abdominal aortic aneurysms, and 5 inflammatory abdominal aneurysms. Using monoclonal anti-CD3 (T cells), anti-CD19 (B cells), anti-CD11c (macrophages), anti-CD4 (T helper cells), and anti-CD8 (T suppressor cells), several distinctions among these groups were found. The amount of inflammatory cell infiltrate was as follows: inflammatory aneurysms more than abdominal aortic aneurysms more than occlusive aortas more than normal aortas. CD3-positive T lymphocytes rarely were found in the adventitia of normal or occlusive aortas. In contrast, abdominal aortic aneurysms and inflammatory aneurysms exhibited most of the CD3-positive infiltrates in the adventitia. CD19-positive B lymphocytes were present mainly in the adventitia of all pathologic tissues. The CD4-positive:CD8-positive ratio was greater in abdominal aortic aneurysms and inflammatory aneurysms than in the other groups, both in the adventitia and in the media of the aortas. CD11c-positive macrophages were present throughout the diseased tissues, often surrounded by lymphoid aggregates; the greatest numbers of macrophages were found in the inflammatory aneurysm group. Our data suggests that the aneurysmal disease may progress from occlusive disease and is accompanied by an increase in chronic inflammatory cells as well as a redistribution of these cell types. Therefore it is suggested that aneurysmal disease may represent an immune-mediated event.
Full text
PDF

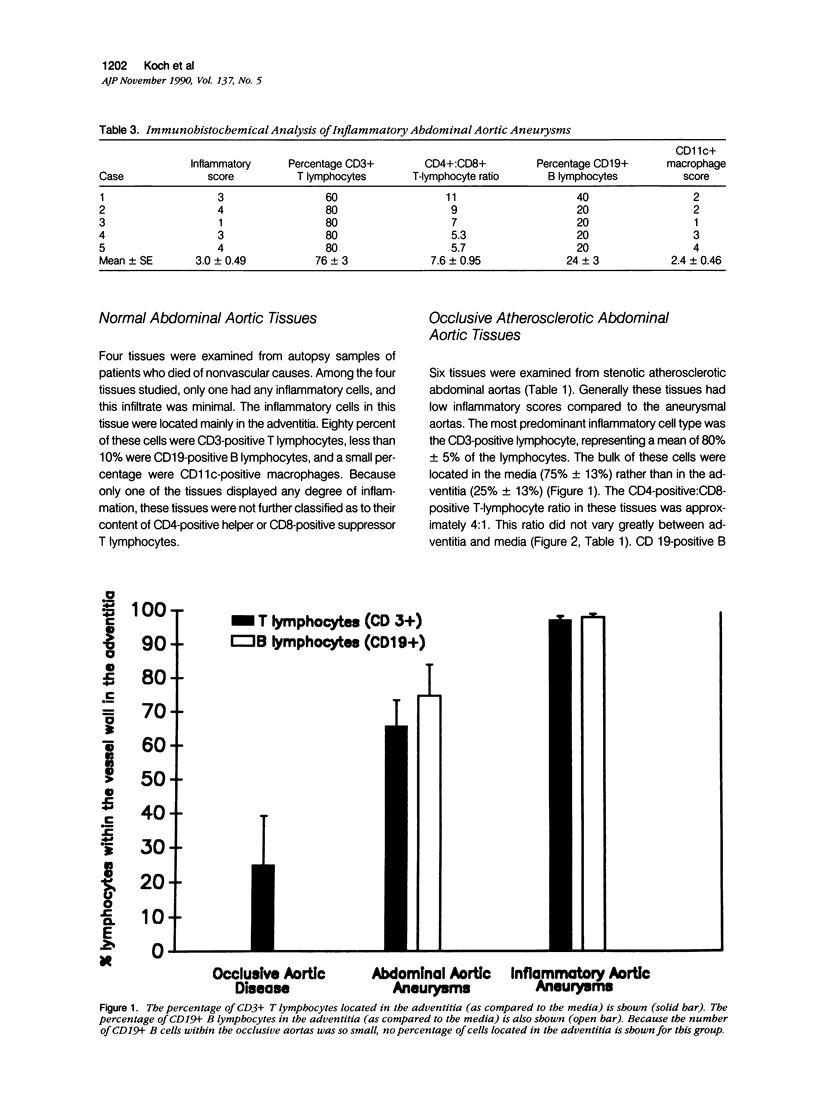
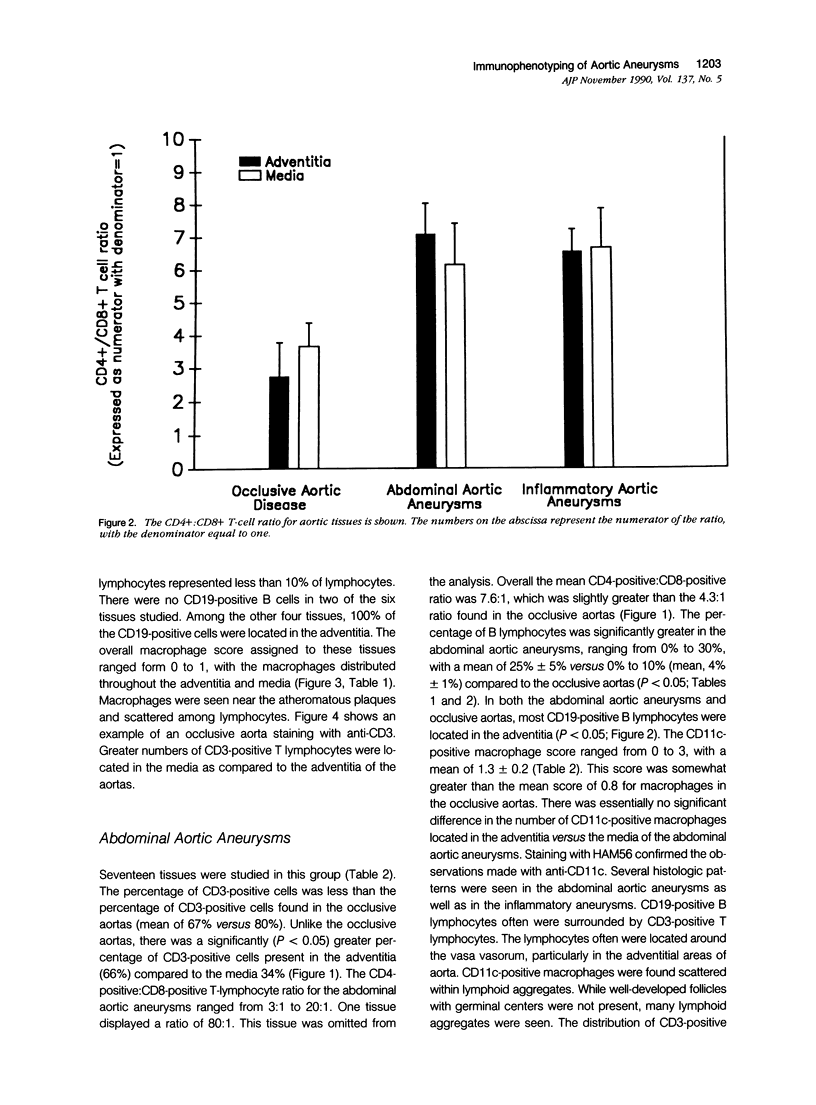
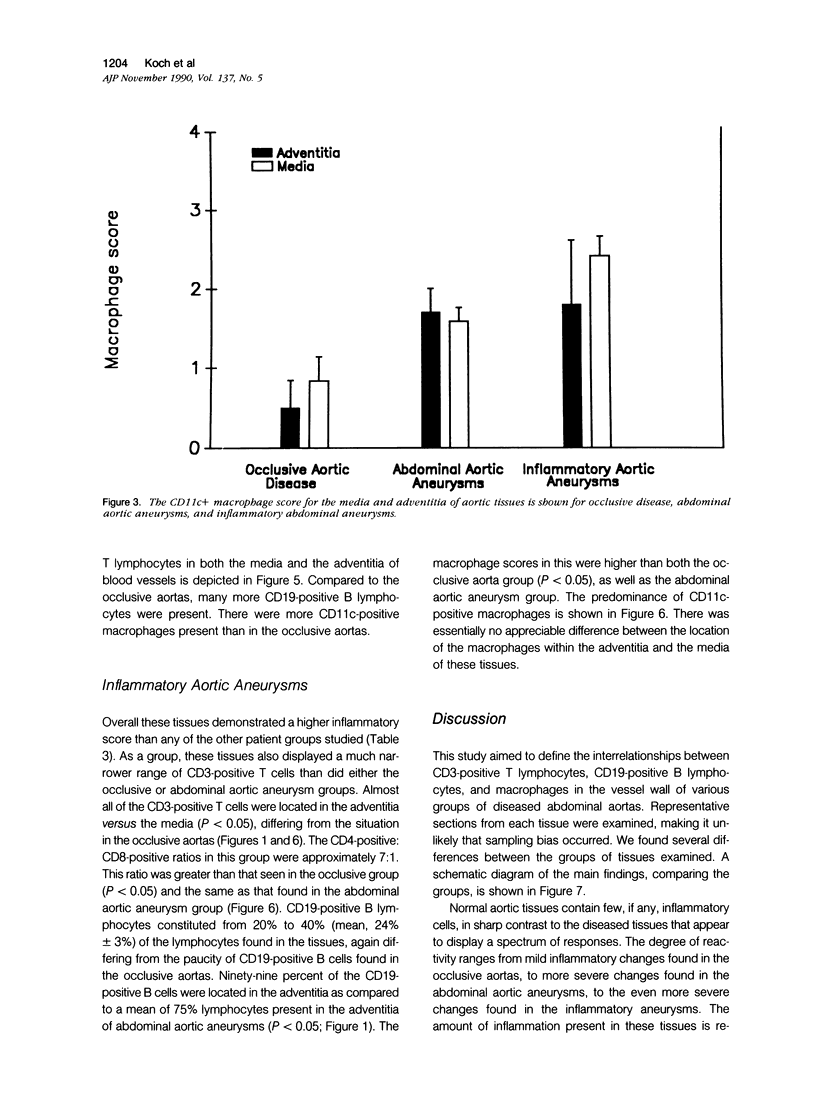


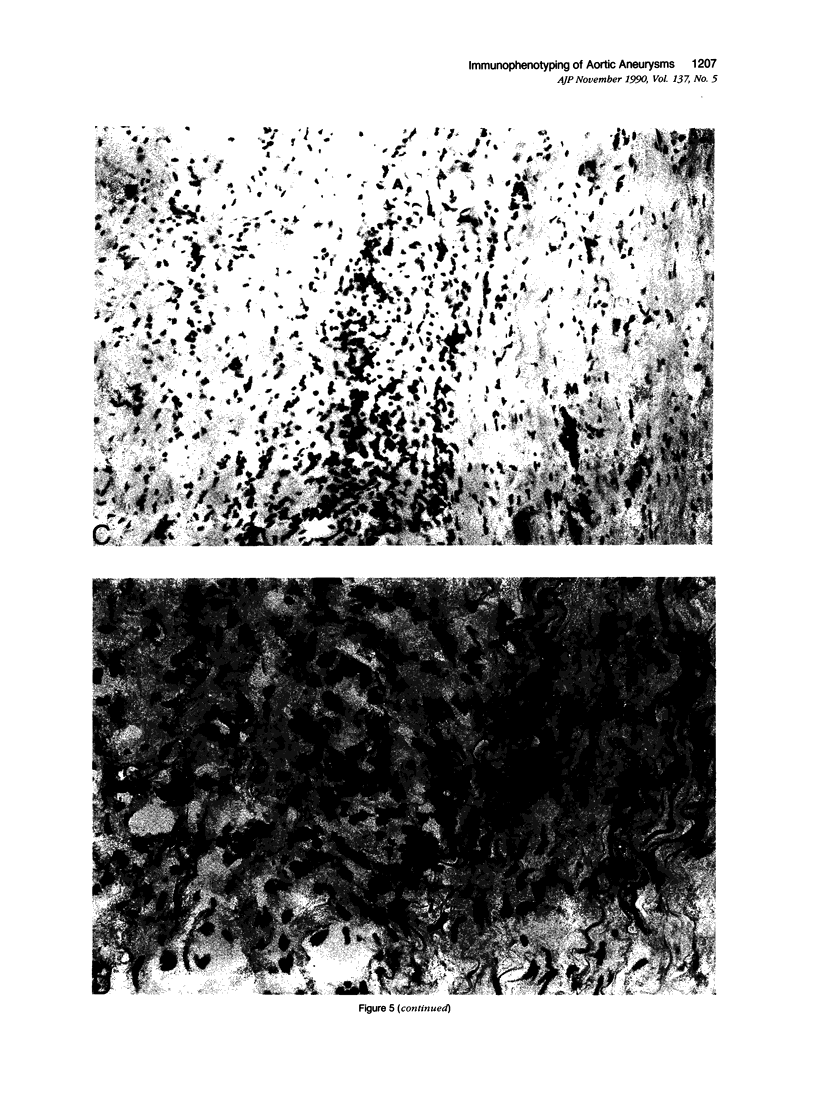
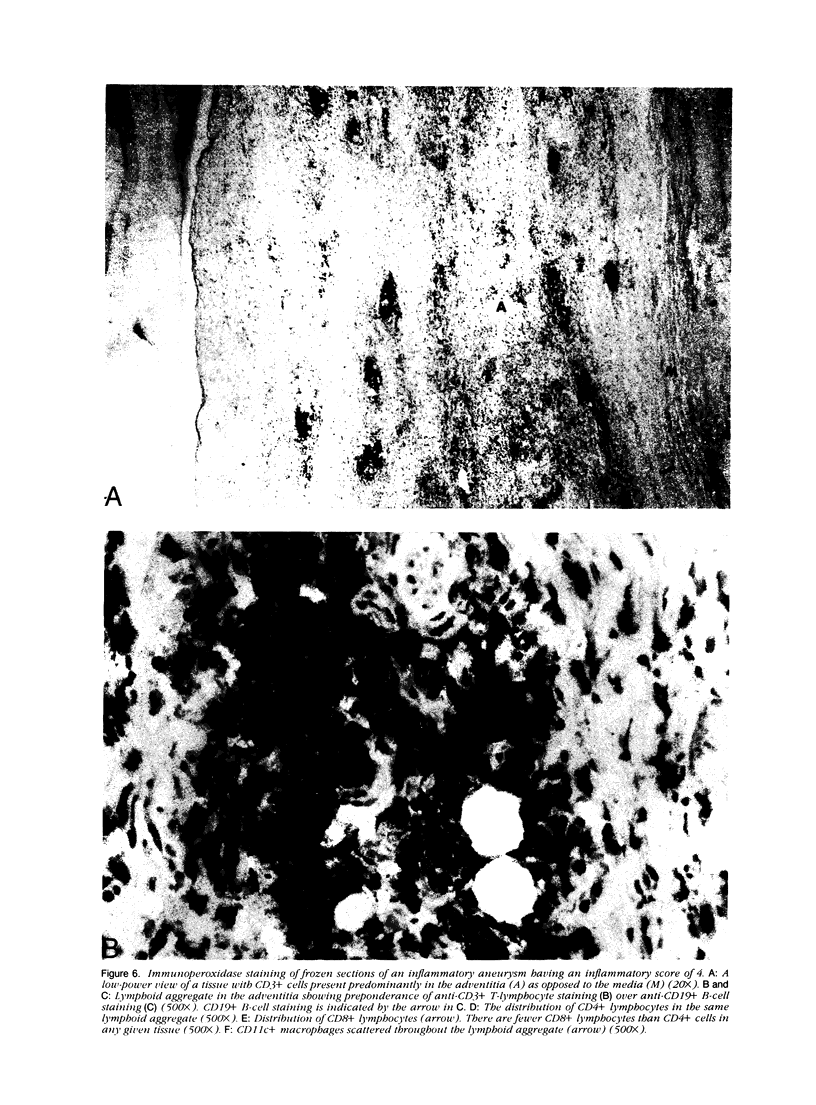
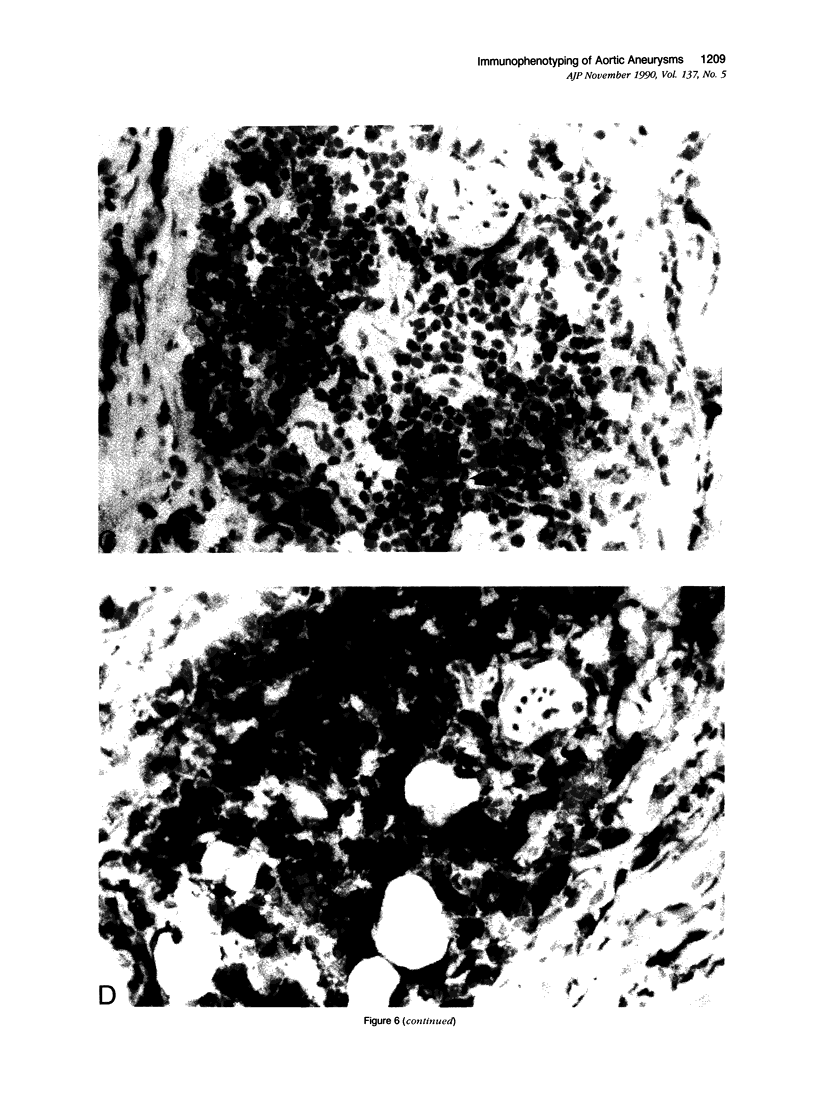
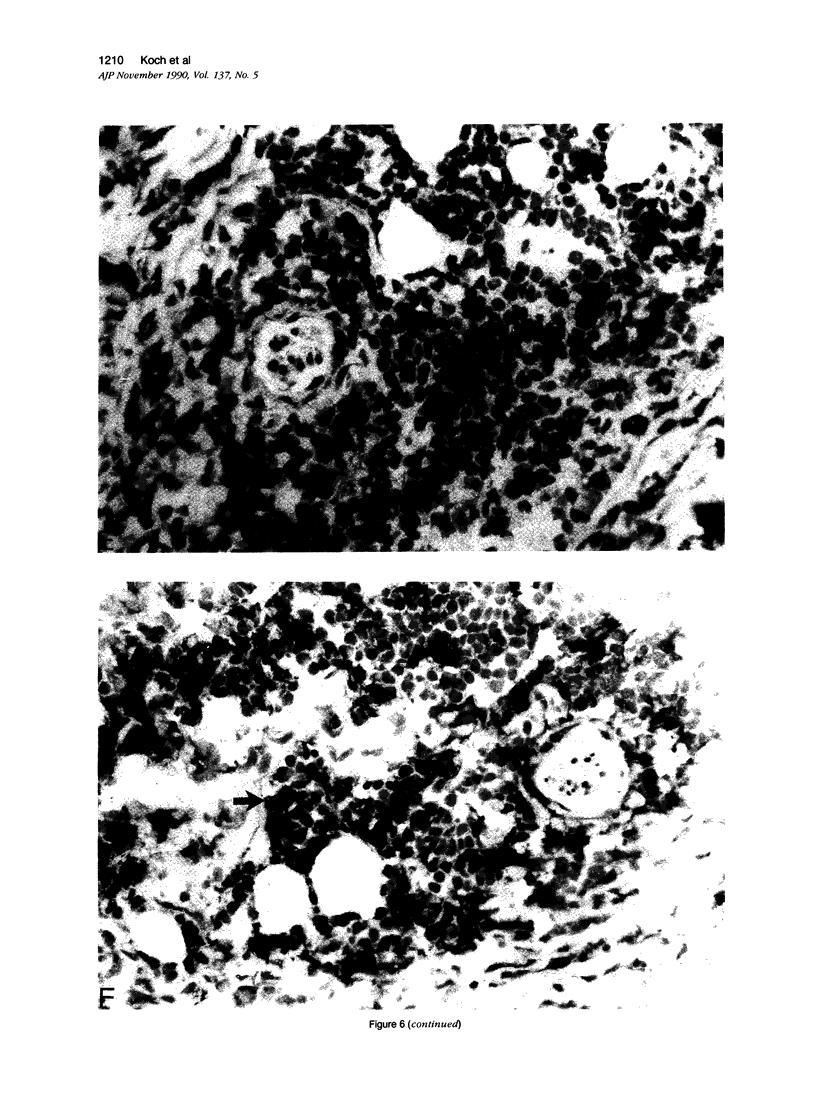


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Cid M. C., Campo E., Ercilla G., Palacin A., Vilaseca J., Villalta J., Ingelmo M. Immunohistochemical analysis of lymphoid and macrophage cell subsets and their immunologic activation markers in temporal arteritis. Influence of corticosteroid treatment. Arthritis Rheum. 1989 Jul;32(7):884–893. [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File T. M., Jr, Barnishan J., Fass R. J. Campylobacter fetus sepsis with mycotic aortic aneurysm. Arch Pathol Lab Med. 1979 Mar;103(3):143–145. [PubMed] [Google Scholar]
- Goldstone J., Malone J. M., Moore W. S. Inflammatory aneurysms of the abdominal aorta. Surgery. 1978 Apr;83(4):425–430. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hamsten A., Norberg R., Björkholm M., de Faire U., Holm G. Antibodies to cardiolipin in young survivors of myocardial infarction: an association with recurrent cardiovascular events. Lancet. 1986 Jan 18;1(8473):113–116. doi: 10.1016/s0140-6736(86)92258-0. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Kral J. G. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand A. 1984 Nov;92(6):429–435. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Lojsthed B., Stemme S., Kocher O., Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988 Aug;72(2-3):135–141. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Hollander W., Colombo M. A., Kirkpatrick B., Paddock J. Soluble proteins in the human atherosclerotic plaque. With spectral reference to immunoglobulins, C3-complement component, alpha 1-antitrypsin and alpha 2-macroglobulin. Atherosclerosis. 1979 Dec;34(4):391–405. doi: 10.1016/0021-9150(79)90064-9. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jarrett F., Darling R. C., Mundth E. D., Austen W. G. Proceeding: Experience with mycotic aneurysms of the abdominal aorta. J Cardiovasc Surg (Torino) 1976 Jan-Feb;17(1):81–81. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Robinson P. G., Radosevich J. A., Pope R. M. Distribution of CD45RA and CD45RO T-lymphocyte subsets in rheumatoid arthritis synovial tissue. J Clin Immunol. 1990 Jul;10(4):192–199. doi: 10.1007/BF00918651. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Mitchinson M. J. Chronic periaortitis and periarteritis. Histopathology. 1984 Jul;8(4):589–600. doi: 10.1111/j.1365-2559.1984.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- O'Brien J. P. A concept of diffuse actinic arteritis. The role of actinic damage to elastin in 'age change' and arteritis of the temporal artery and in polymyalgia rheumatica. Br J Dermatol. 1978 Jan;98(1):1–13. doi: 10.1111/j.1365-2133.1978.tb07327.x. [DOI] [PubMed] [Google Scholar]
- Rizzo R. J., McCarthy W. J., Dixit S. N., Lilly M. P., Shively V. P., Flinn W. R., Yao J. S. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989 Oct;10(4):365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. G., Dent D. M. Inflammatory variant of abdominal atherosclerotic aneurysm. Arch Pathol Lab Med. 1981 Aug;105(8):409–413. [PubMed] [Google Scholar]
- SCHWARTZ C. J., MITCHELL J. R. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962 Jul;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- SOMMERVILLE R. L., ALLEN E. V., EDWARDS J. E. Bland and infected arteriosclerotic abdominal aortic aneurysms: a clinicopathologic study. Medicine (Baltimore) 1959 Sep;38:207–221. doi: 10.1097/00005792-195909000-00001. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Sprague E. A., Kelley J. L., Suenram C. A., Graves D. T., Rozek M. M., Edwards E. H., Delgado R. Monocyte-macrophage participation in atherogenesis: inflammatory components of pathogenesis. Semin Thromb Hemost. 1986 Apr;12(2):79–86. doi: 10.1055/s-2007-1003539. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Walker D. I., Bloor K., Williams G., Gillie I. Inflammatory aneurysms of the abdominal aorta. Br J Surg. 1972 Aug;59(8):609–614. doi: 10.1002/bjs.1800590807. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Bentz van de Berg D., van der Loos C. M., Becker A. E. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989 Aug;61(2):166–170. [PubMed] [Google Scholar]