Abstract
Various cholestatic liver diseases are accompanied by a striking increase in the number of bile ductules. This so-called ductular reaction is thought to arise both from ductular metaplasia of hepatocytes and from proliferation of pre-existing bile ductules. Previous studies have shown that these reactive bile ductules differ from their normal counterpart in enzyme and immunohistochemical make-up. Using monoclonal antibodies directed to neuroendocrine markers and immunohistochemistry, we found that reactive bile ductules in cholestatic liver disease display neuroendocrine features. In all cases of primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), extrahepatic obstruction, and acute hepatitis A, reactive bile ductules expressed the neural cell adhesion molecule (N-CAM) and reacted with monoclonal antibody A2B5. Both N-CAM and the ganglioside, recognized by A2B5, are restricted to neuroendocrine cells and tissues. In all but four of these cases, the same bile ductules expressed chromogranin-A, present in the matrix of neuroendocrine granules. Furthermore, in three cases of longstanding cholestasis, scattered periportal hepatocytes expressed chromogranin-A but not N-CAM. Other neuroendocrine markers such as neuron-specific enolase, synaptophysin, or myelin-associated glycoprotein were lacking from both bile ductules and hepatocytes. The neuroendocrine phenotype of bile ductules and hepatocytes was confirmed on electronmicroscopy, demonstrating various numbers of dense-cored, neuroendocrine granules near the peripheral cell membrane in bile ductules as well as in cells intermediate between hepatocytes and bile ductular cells. In 10 cases of normal liver tissue without ductular reaction, bile ductules lacked neuroendocrine markers except in two cases in which very weak reactivity for chromogranin-A was observed. These findings illustrate the presence of a new, neuroendocrine cell type that emerges in the liver during cholestasis. Elucidation of the significance of the neuroendocrine substance(s) produced in the dense cored granules of reactive bile ductules awaits their isolation and characterization. We can speculate that this molecule plays an autocrine or paracrine regulatory role in the process of ductular metaplasia of hepatocytes or growth of bile ductules.
Full text
PDF
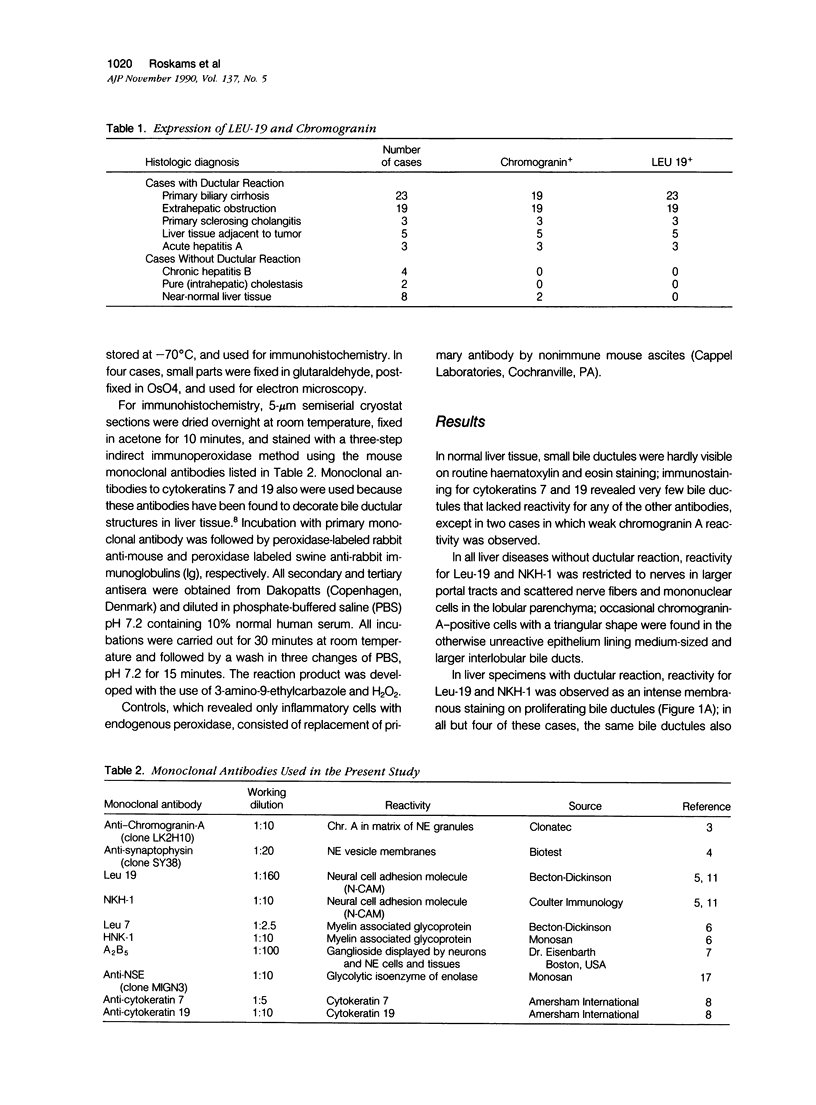


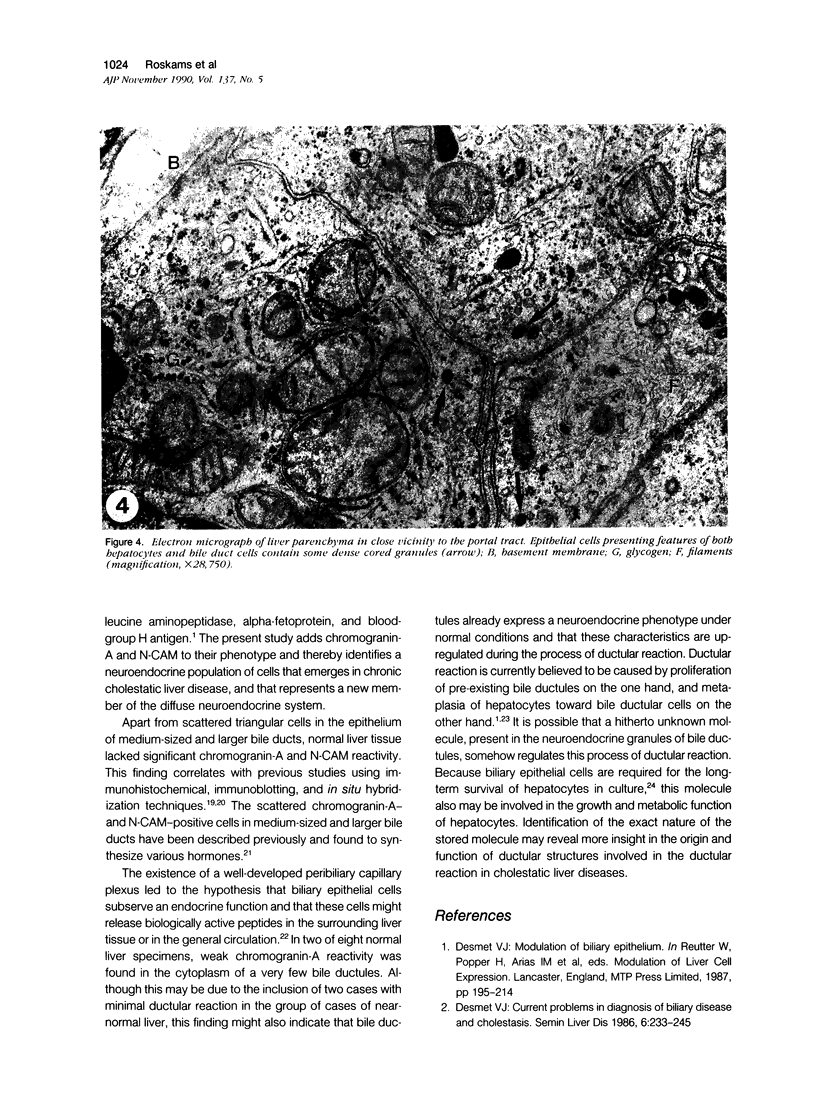

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop A. E., Power R. F., Polak J. M. Markers for neuroendocrine differentiation. Pathol Res Pract. 1988 Apr;183(2):119–128. doi: 10.1016/s0344-0338(88)80040-2. [DOI] [PubMed] [Google Scholar]
- Clement B., Guguen-Guillouzo C., Campion J. P., Glaise D., Bourel M., Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984 May-Jun;4(3):373–380. doi: 10.1002/hep.1840040305. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Chuong C. M., Edelman G. M. Expression sequences of cell adhesion molecules. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6942–6946. doi: 10.1073/pnas.82.20.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet V. J. Current problems in diagnosis of biliary disease and cholestasis. Semin Liver Dis. 1986 Aug;6(3):233–245. doi: 10.1055/s-2008-1040606. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Miyazawa Y., Imoto M., Koyama Y., Nakano I., Nagura H., Kato K. In situ distribution of enolase isozymes in chronic liver disease. Am J Gastroenterol. 1989 Jun;84(6):601–605. [PubMed] [Google Scholar]
- Kurumaya H., Ohta G., Nakanuma Y. Endocrine cells in the intrahepatic biliary tree in normal livers and hepatolithiasis. Arch Pathol Lab Med. 1989 Feb;113(2):143–147. [PubMed] [Google Scholar]
- Langley O. K., Aletsee-Ufrecht M. C., Grant N. J., Gratzl M. Expression of the neural cell adhesion molecule NCAM in endocrine cells. J Histochem Cytochem. 1989 Jun;37(6):781–791. doi: 10.1177/37.6.2723399. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Testi R., Bindl J., Phillips J. H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989 Jun 1;169(6):2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Caillaud J. M., Carlu C., Tursz T. HNK-1 antibody detects an antigen expressed on neuroectodermal cells. J Exp Med. 1983 Nov 1;158(5):1775–1780. doi: 10.1084/jem.158.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Iacangelo A., Eiden L. E., Cano M., Jin L., Grimes M. Chromogranin A and B messenger ribonucleic acids in pituitary and other normal and neoplastic human endocrine tissues. Lab Invest. 1989 Apr;60(4):548–556. [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Fields K. Detection of chromogranins A and B in endocrine tissues with radioactive and biotinylated oligonucleotide probes. Am J Surg Pathol. 1990 Jan;14(1):35–43. doi: 10.1097/00000478-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Wilson B. S. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983 Nov 11;222(4624):628–630. doi: 10.1126/science.6635661. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Owens G. C., Prediger E. A., Crossin K. L., Cunningham B. A., Edelman G. M. Cell surface modulation of the neural cell adhesion molecule resulting from alternative mRNA splicing in a tissue-specific developmental sequence. J Cell Biol. 1986 Oct;103(4):1431–1439. doi: 10.1083/jcb.103.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani O. The peribiliary portal system in the rabbit liver. Arch Histol Jpn. 1979 Apr;42(2):153–167. doi: 10.1679/aohc1950.42.153. [DOI] [PubMed] [Google Scholar]
- Popper H. The relation of mesenchymal cell products to hepatic epithelial systems. Prog Liver Dis. 1990;9:27–38. [PubMed] [Google Scholar]
- SCHAFFNER F., POPPER H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961 Apr;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- Simon J. P., Aunis D. Biochemistry of the chromogranin A protein family. Biochem J. 1989 Aug 15;262(1):1–13. doi: 10.1042/bj2620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz W., Schachner M. Adhesion of neural cells to extracellular matrix constituents. Involvement of glycosaminoglycans and cell adhesion molecules. Brain Res. 1988 Oct 1;471(2):225–234. doi: 10.1016/0165-3806(88)90101-0. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- van Eyken P., Sciot R., van Damme B., de Wolf-Peeters C., Desmet V. J. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]