Abstract
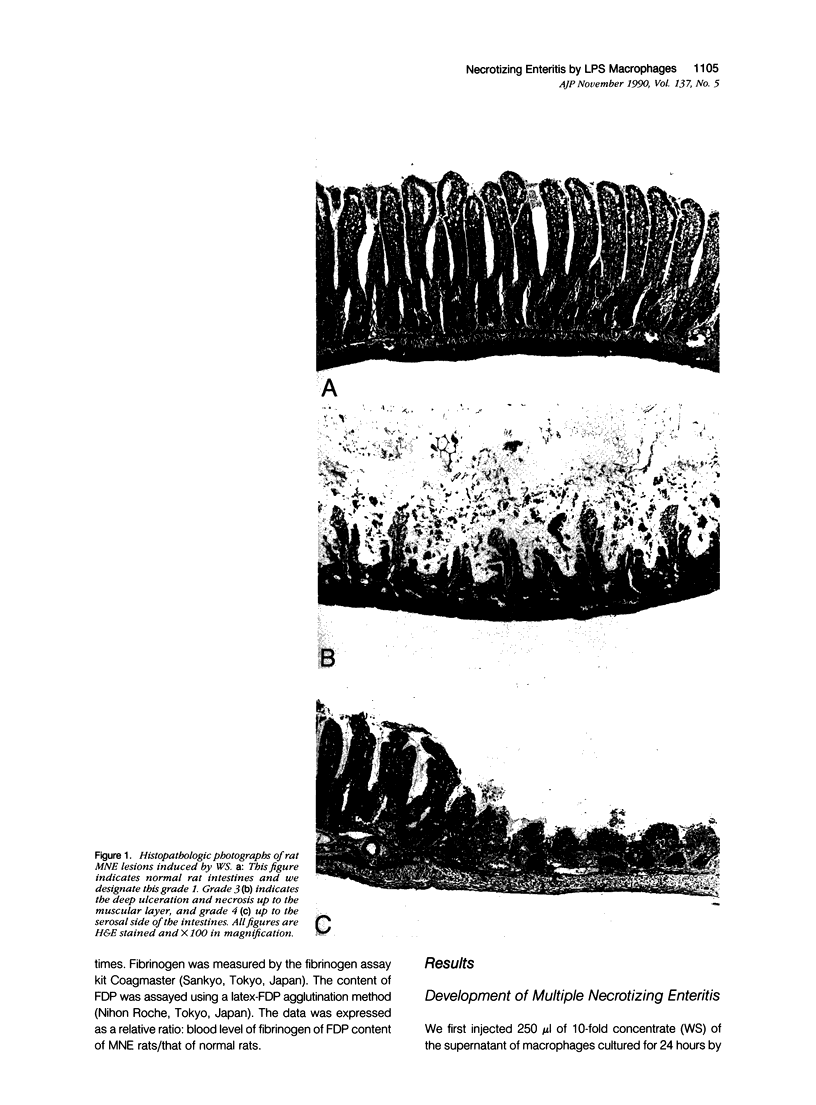
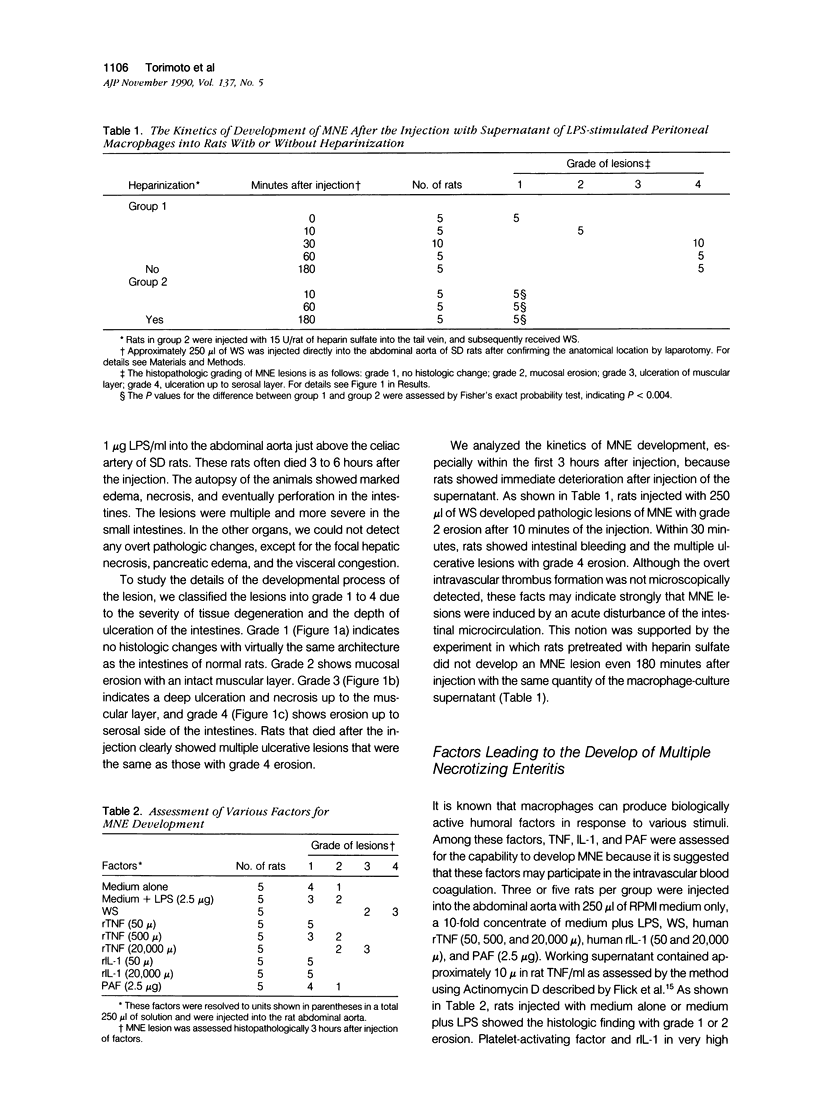
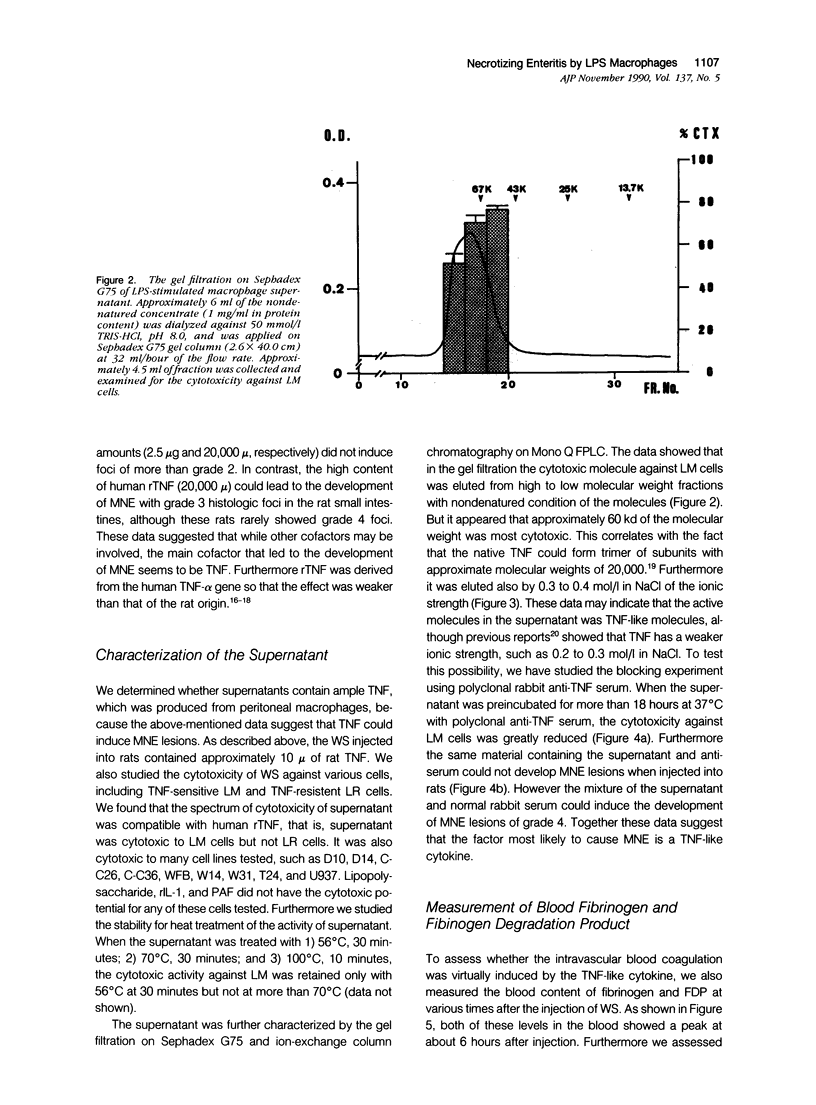
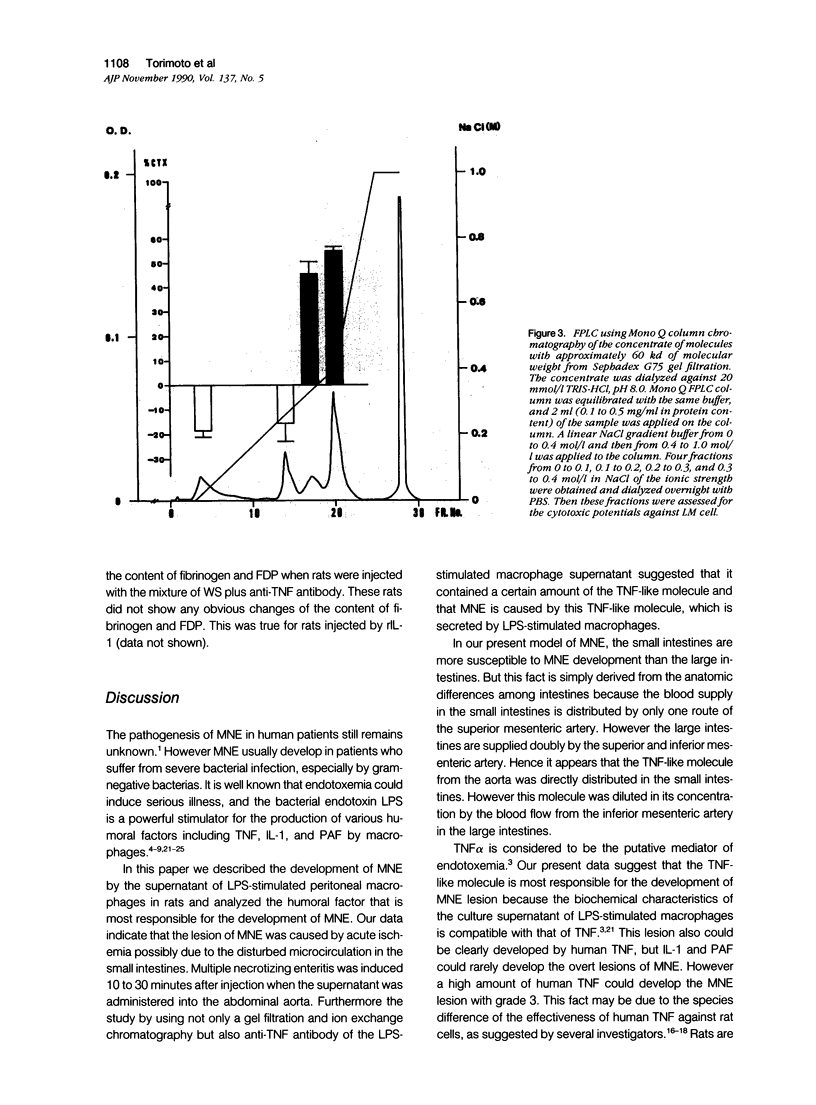
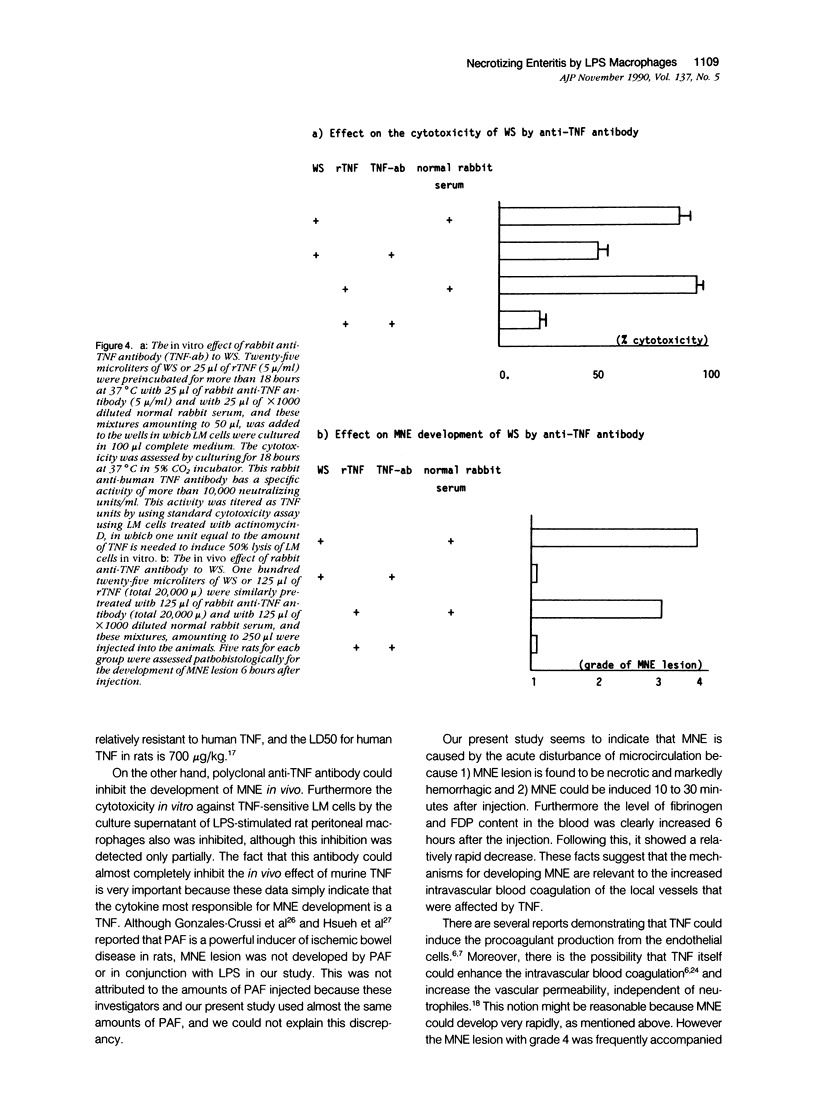
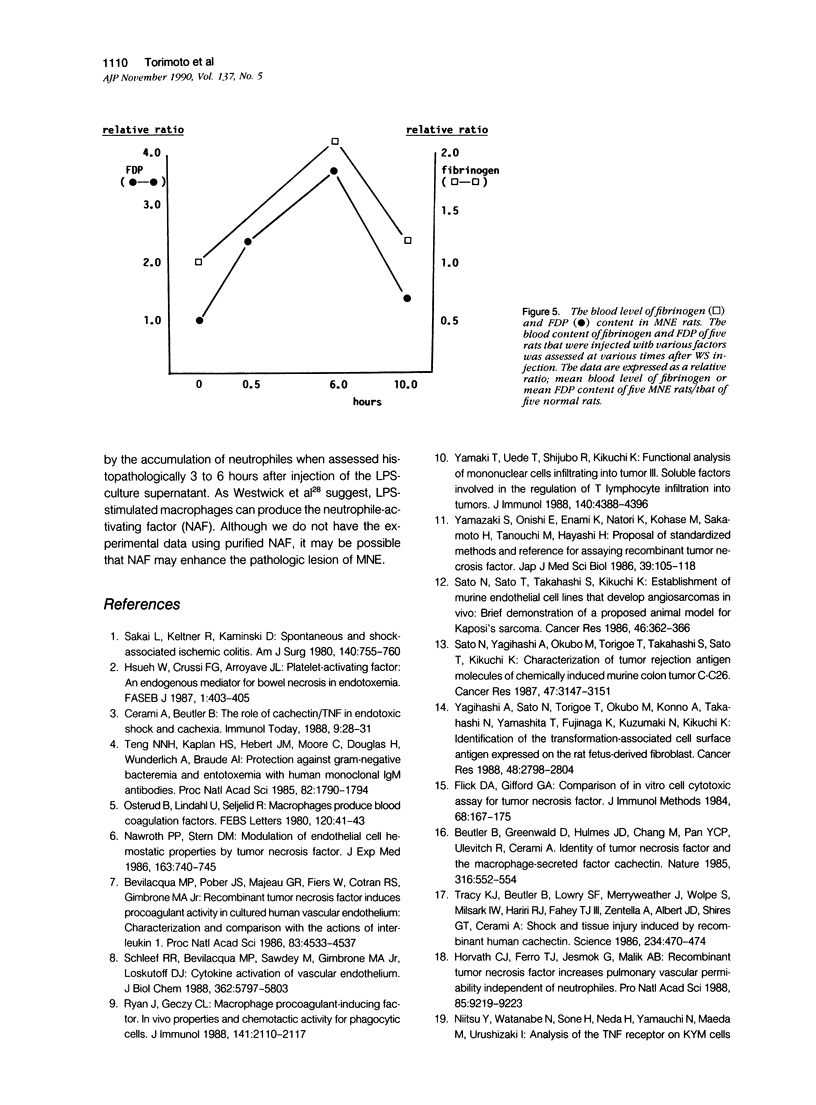
We report the development of an animal model of multiple necrotizing enteritis (MNE) in rats. When rats were injected directly with a culture supernatant of lipopolysaccharide (LPS)-stimulated rat peritoneal macrophages into the abdominal aorta, the overt pathologic lesions of MNE developed within 30 minutes after injection. The rats showed an elevated level of blood fibrinogen degradation product content even 30 minutes after injection. Furthermore the rats that were pretreated intravenously with heparin sulfate did not develop MNE, indicating the acute disturbances of blood microcirculation in the intestine. Multiple necrotizing enteritis was developed also by the injection with recombinant tumor necrosis factor (rTNF) but rarely was observed with even a high dose of recombinant interleukin-1 (rIL-1) or platelet-activating factor (PAF). The supernatant was cytotoxic in vitro to TNF-susceptible LM and many other cells but was less cytotoxic to the TNF-resistant LR line. Partial purification of the supernatant suggested that the supernatant contained a cytokine that has biochemical features of TNF. Furthermore polyclonal anti-TNF antibody could inhibit not only the cytotoxicity in vitro but also MNE development in vivo by this factor. These data strongly indicate that MNE possibly could be caused by a TNF-like cytokine produced by macrophages that are stimulated by the endotoxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett J., Gerlach H., Nawroth P., Steinberg S., Godman G., Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989 Jun 1;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender D. E., Edelbaum D. Inhibition by IL-1 of endothelial cell activation induced by tumor necrosis factor or lymphotoxin. J Immunol. 1988 Nov 1;141(9):3111–3116. [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983 Jul;112(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Haranaka K., Satomi N., Sakurai A., Nariuchi H. Purification and partial amino acid sequence of rabbit tumor necrosis factor. Int J Cancer. 1985 Sep 15;36(3):395–400. [PubMed] [Google Scholar]
- Horvath C. J., Ferro T. J., Jesmok G., Malik A. B. Recombinant tumor necrosis factor increases pulmonary vascular permeability independent of neutrophils. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9219–9223. doi: 10.1073/pnas.85.23.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., Gonzalez-Crussi F., Arroyave J. L. Platelet-activating factor-induced ischemic bowel necrosis. An investigation of secondary mediators in its pathogenesis. Am J Pathol. 1986 Feb;122(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., González-Crussi F., Arroyave J. L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987 Nov;1(5):403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Havell E. A. The antitumor function of tumor necrosis factor (TNF) II. Analysis of the role of endogenous TNF in endotoxin-induced hemorrhagic necrosis and regression of an established sarcoma. J Exp Med. 1988 Mar 1;167(3):1086–1099. doi: 10.1084/jem.167.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Lindahl U., Seljelid R. Macrophages produce blood coagulation factors. FEBS Lett. 1980 Oct 20;120(1):41–43. doi: 10.1016/0014-5793(80)81041-6. [DOI] [PubMed] [Google Scholar]
- Ryan J., Geczy C. L. Macrophage procoagulant-inducing factor. In vivo properties and chemotactic activity for phagocytic cells. J Immunol. 1988 Sep 15;141(6):2110–2117. [PubMed] [Google Scholar]
- Sakai L., Keltner R., Kaminski D. Spontaneous and shock-associated ischemic colitis. Am J Surg. 1980 Dec;140(6):755–760. doi: 10.1016/0002-9610(80)90111-7. [DOI] [PubMed] [Google Scholar]
- Sato N., Sato T., Takahashi S., Kikuchi K. Establishment of murine endothelial cell lines that develop angiosarcomas in vivo: brief demonstration of a proposed animal model for Kaposi's sarcoma. Cancer Res. 1986 Jan;46(1):362–366. [PubMed] [Google Scholar]
- Sato N., Yagihashi A., Okubo M., Torigoe T., Takahashi S., Sato T., Kikuchi K. Characterization of tumor rejection antigen molecules of chemically induced murine colon tumor C-C26. Cancer Res. 1987 Jun 15;47(12):3147–3151. [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Yagihashi A., Sato N., Torigoe T., Okubo M., Konno A., Takahashi N., Yamashita T., Fujinaga K., Kuzumaki N., Kikuchi K. Identification of the transformation-associated cell surface antigen expressed on the rat fetus-derived fibroblast. Cancer Res. 1988 May 15;48(10):2798–2804. [PubMed] [Google Scholar]
- Yamaki T., Uede T., Shijubo N., Kikuchi K. Functional analysis of mononuclear cells infiltrating into tumors. III. Soluble factors involved in the regulation of T lymphocyte infiltration into tumors. J Immunol. 1988 Jun 15;140(12):4388–4396. [PubMed] [Google Scholar]
- Yamazaki S., Onishi E., Enami K., Natori K., Kohase M., Sakamoto H., Tanouchi M., Hayashi H. Proposal of standardized methods and reference for assaying recombinant human tumor necrosis factor. Jpn J Med Sci Biol. 1986 Jun;39(3):105–118. doi: 10.7883/yoken1952.39.105. [DOI] [PubMed] [Google Scholar]




