Abstract
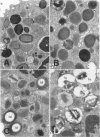
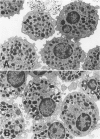
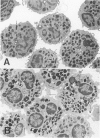
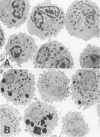
Hypodense eosinophils were obtained from two patients with the idiopathic hyperosinophilic syndrome (IHES), and hypodense eosinophils were derived by culturing normodense human eosinophils from control donors in the presence of endothelial cells alone, granulocyte/macrophage-colony-stimulating factor (GM-CSF) alone, or GM-CSF and fibroblasts. These eosinophils were examined ultrastructurally and stereologically for alterations in the volume density (Vv) of their electron-dense granules, the Vv of their lucent granules, the Vv of their lipid droplets, the numerical density of their granules with respect to cytoplasm (Nv), and the plasma membrane surface area-to-cell volume ratio (Sv) that might account for their decreased sedimentation density. The hypodense eosinophils that were obtained from the two patients with IHES exhibited a one-third reduction in granule Vv relative to normodense eosinophils from control donors, primarily because of a decrease in granule size. The culture-derived hypodense eosinophils exhibited 10% to 16% decreases in their granule Vv, significant increases in their lucent granules, and a approximately 7.5% decrease in their Sv. Calculation of the cell volume from cross-sectional area measurements showed that the eosinophils that had been cocultured with fibroblasts in the presence of GM-CSF increased their volume by approximately 15%. The eosinophils that had been cocultured with endothelial cells exocytosed some of their granules. In conclusion, a composite of factors including cell swelling, a decrease in the volume of the cytoplasm occupied by granules, and an increase in granule lucency contributes to the hypodense phenotype in vitro, but only cell swelling and hypogranulation are seen in cells from patients with IHES. The latter could reflect the response of 'primed' hypodense eosinophils in vivo to pertinent tissue ligands.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Simone C., Donneli G., Meli D., Rosati F., Sorice F. Human eosinophils and parasitic diseases. II. Characterization of two cell fractions isolated at different densities. Clin Exp Immunol. 1982 Apr;48(1):249–255. [PMC free article] [PubMed] [Google Scholar]
- Greenspan P., Mayer E. P., Fowler S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985 Mar;100(3):965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985 Feb;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
- Kajita T., Yui Y., Mita H., Taniguchi N., Saito H., Mishima T., Shida T. Release of leukotriene C4 from human eosinophils and its relation to the cell density. Int Arch Allergy Appl Immunol. 1985;78(4):406–410. doi: 10.1159/000233922. [DOI] [PubMed] [Google Scholar]
- Lamas A. M., Marcotte G. V., Schleimer R. P. Human endothelial cells prolong eosinophil survival. Regulation by cytokines and glucocorticoids. J Immunol. 1989 Jun 1;142(11):3978–3984. [PubMed] [Google Scholar]
- Lucey D. R., Dorsky D. I., Nicholson-Weller A., Weller P. F. Human eosinophils express CD4 protein and bind human immunodeficiency virus 1 gp120. J Exp Med. 1989 Jan 1;169(1):327–332. doi: 10.1084/jem.169.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey D. R., Nicholson-Weller A., Weller P. F. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Jr, Rothenberg M. E., Silberstein D. S., Gasson J. C., Stevens R. L., Austen K. F., Soberman R. J. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987 Jul 1;166(1):129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Rothenberg M. E., Petersen J., Weller P. F., Silberstein D., Sheffer A. L., Stevens R. L., Soberman R. J., Austen K. F. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989 Jul 1;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M. S., Gleich G. J., Dunnette S. L., Fukuda T. Ultrastructural study of eosinophils from patients with the hypereosinophilic syndrome: a morphological basis of hypodense eosinophils. Blood. 1988 Mar;71(3):780–785. [PubMed] [Google Scholar]
- Pinkston P., Vijayan V. K., Nutman T. B., Rom W. N., O'Donnell K. M., Cornelius M. J., Kumaraswami V., Ferrans V. J., Takemura T., Yenokida G. Acute tropical pulmonary eosinophilia. Characterization of the lower respiratory tract inflammation and its response to therapy. J Clin Invest. 1987 Jul;80(1):216–225. doi: 10.1172/JCI113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prin L., Capron M., Tonnel A. B., Bletry O., Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72(4):336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- Prin L., Charon J., Capron M., Gosset P., Taelman H., Tonnel A. B., Capron A. Heterogeneity of human eosinophils. II. Variability of respiratory burst activity related to cell density. Clin Exp Immunol. 1984 Sep;57(3):735–742. [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Soberman R. J., Austen K. F., Stevens R. L. Eosinophils cocultured with endothelial cells have increased survival and functional properties. Science. 1987 Aug 7;237(4815):645–647. doi: 10.1126/science.3110954. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Petersen J., Stevens R. L., Silberstein D. S., McKenzie D. T., Austen K. F., Owen W. F., Jr IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989 Oct 1;143(7):2311–2316. [PubMed] [Google Scholar]
- Shaw R. J., Walsh G. M., Cromwell O., Moqbel R., Spry C. J., Kay A. B. Activated human eosinophils generate SRS-A leukotrienes following IgG-dependent stimulation. Nature. 1985 Jul 11;316(6024):150–152. doi: 10.1038/316150a0. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Tai P. C. Studies on blood eosinophils. II. Patients with Löffler's cardiomyopathy. Clin Exp Immunol. 1976 Jun;24(3):423–434. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Ackerman S. J., Spry C. J., Dunnette S., Olsen E. G., Gleich G. J. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987 Mar 21;1(8534):643–647. doi: 10.1016/s0140-6736(87)90412-0. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin Exp Immunol. 1976 Jun;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. The mechanisms which produce vacuolated and degranulated eosinophils. Br J Haematol. 1981 Oct;49(2):219–226. doi: 10.1111/j.1365-2141.1981.tb07218.x. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Dvorak A. M. Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood. 1985 May;65(5):1269–1274. [PubMed] [Google Scholar]
- Winqvist I., Olofsson T., Olsson I., Persson A. M., Hallberg T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology. 1982 Nov;47(3):531–539. [PMC free article] [PubMed] [Google Scholar]