Abstract
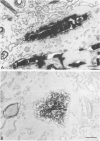
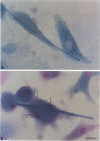
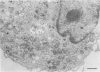
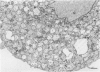
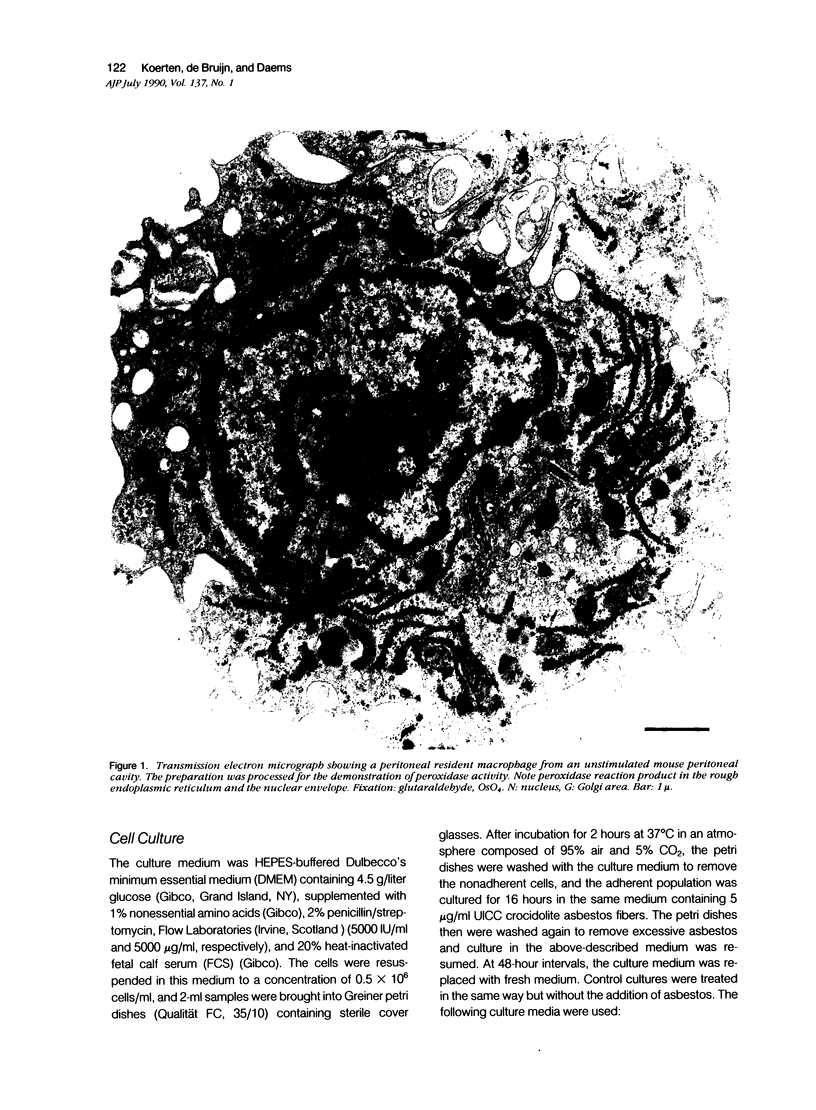
For studies on the mechanism of asbestos body formation, Union Internationale Contre Cancer (UICC) crocidolite asbestos fibers were added to a culture of mouse peritoneal macrophages. Small asbestos fibers were totally ingested by the macrophages, but fibers too long to be taken up completely remained as a consequence extracellular. These long asbestos fibers became the basis for asbestos body formation. The basic mechanism underlying asbestos body formation was found to be the exocytotic activity of macrophages. The number of iron-rich inclusion bodies was dependent on the availability of iron in the culture media, and the same holds for the amount of iron in the asbestos body coat. This means that asbestos body formation is a phenomenon that occurs accidentally when macrophages come into contact with long fibers in an iron-rich environment. A time-dependent increase in the number, average size, and rate of segmentation of the asbestos bodies was observed. The present report is the first to describe asbestos body formation in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach O., Conston A. S., Garfinkel L., Parks V. R., Kaslow H. D., Hammond E. C. Presence of asbestos bodies in organs other than the lung. Chest. 1980 Feb;77(2):133–137. doi: 10.1378/chest.77.2.133. [DOI] [PubMed] [Google Scholar]
- Bey E., Harington J. S. Cytotoxic effects of some mineral dusts on Syrian hamster peritoneal macrophages. J Exp Med. 1971 May 1;133(5):1149–1169. doi: 10.1084/jem.133.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botham S. K., Holt P. F. The mechanism of formation of asbestos bodies. J Pathol Bacteriol. 1968 Oct;96(2):443–453. doi: 10.1002/path.1700960223. [DOI] [PubMed] [Google Scholar]
- Chamberlain M. Effect of mineral dusts on metabolic cooperation between Chinese hamster V79 cells in vitro. Environ Health Perspect. 1983 Sep;51:5–9. doi: 10.1289/ehp.83515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daems W. T., Koerten H. K. The effects of various stimuli on the cellular composition of peritoneal exudates in the mouse. Cell Tissue Res. 1978 Jun 26;190(1):47–60. doi: 10.1007/BF00210035. [DOI] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Ackerman J., Butterfield A., Williams S. Asbestos induces selective release of lysosomal enzymes from mononuclear phagocytes. Nature. 1974 Oct 4;251(5474):423–425. doi: 10.1038/251423a0. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Gross P., De Treville R. T. "Ferruginous bodies" in guinea pigs. Fine structure produced experimentally from minerals other than asbestos. Arch Pathol. 1970 Apr;89(4):364–373. [PubMed] [Google Scholar]
- Elferink J. G., Deierkauf M., Kramps J. A., Koerten H. K. An activating and cytotoxic effect of asbestos on polymorphonuclear leukocytes. Agents Actions. 1989 Jan;26(1-2):213–215. doi: 10.1007/BF02126614. [DOI] [PubMed] [Google Scholar]
- Gross P., de Treville R. T., Cralley L. J., Davis J. M. Pulmonary ferruginous bodies. Development in response to filamentous dusts and a method of isolation and concentration. Arch Pathol. 1968 May;85(5):539–546. [PubMed] [Google Scholar]
- Harrison P. T., Heath J. C. Asbestos bodies in rat lung following intratracheal instillation of chrysotile. Lab Anim. 1986 Apr;20(2):132–137. doi: 10.1258/002367786780865223. [DOI] [PubMed] [Google Scholar]
- Hulstaert C. E., Kalicharan D., Hardonk M. J. Cytochemical demonstration of phosphatases in the rat liver by a cerium-based method in combination with osmium tetroxide and potassium ferrocyanide postfixation. Histochemistry. 1983;78(1):71–79. doi: 10.1007/BF00491113. [DOI] [PubMed] [Google Scholar]
- Kagan E., Oghiso Y., Hartmann D. P. Enhanced release of a chemoattractant for alveolar macrophages after asbestos inhalation. Am Rev Respir Dis. 1983 Oct;128(4):680–687. doi: 10.1164/arrd.1983.128.4.680. [DOI] [PubMed] [Google Scholar]
- Kanazawa K., Birbeck M. S., Carter R. L., Roe F. J. Migration of asbestos fibres from subcutaneous injection sites in mice. Br J Cancer. 1970 Mar;24(1):96–106. doi: 10.1038/bjc.1970.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerten H. K., Hazekamp J., Kroon M., Daems W. T. Asbestos body formation and iron accumulation in mouse peritoneal granulomas after the introduction of crocidolite asbestos fibers. Am J Pathol. 1990 Jan;136(1):141–157. [PMC free article] [PubMed] [Google Scholar]
- Lemaire I., Rola-Pleszczynski M., Bégin R. Asbestos exposure enhances the release of fibroblast growth factor by sheep alveolar macrophages. J Reticuloendothel Soc. 1983 Apr;33(4):275–285. [PubMed] [Google Scholar]
- Mace M. L., Jr, McLemore T. L., Roggli V., Brinkley B. R., Greenberg S. D. Scanning electron microscopic examination of human asbestos bodies. Cancer Lett. 1980 Apr;9(2):95–104. doi: 10.1016/0304-3835(80)90112-3. [DOI] [PubMed] [Google Scholar]
- Mace M. L., Jr, McLemore T. L., Roggli V., Brinkley B. R., Greenberg S. D. Scanning electron microscopic examination of human asbestos bodies. Cancer Lett. 1980 Apr;9(2):95–104. doi: 10.1016/0304-3835(80)90112-3. [DOI] [PubMed] [Google Scholar]
- Morgan A., Holmes A. The enigmatic asbestos body: its formation and significance in asbestos-related disease. Environ Res. 1985 Dec;38(2):283–292. doi: 10.1016/0013-9351(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Nadeau D., Fouquette-Couture L., Paradis D., Khorami J., Lane D., Dunnigan J. Cytotoxicity of respirable dusts from industrial minerals: comparison of two naturally occurring and two man-made silicates. Drug Chem Toxicol. 1987;10(1-2):49–86. doi: 10.3109/01480548709042583. [DOI] [PubMed] [Google Scholar]
- Paterour M. J., Bignon J., Jaurand M. C. In vitro transformation of rat pleural mesothelial cells by chrysotile fibres and/or benzo[a]pyrene. Carcinogenesis. 1985 Apr;6(4):523–529. doi: 10.1093/carcin/6.4.523. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Churg J. Structure and development of the asbestos body. Am J Pathol. 1969 Apr;55(1):79–107. [PMC free article] [PubMed] [Google Scholar]
- Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988 Jun;(231):239–271. [PubMed] [Google Scholar]
- de Bruijn W. C. Glycogen, its chemistry and morphologic appearance in the electron microscope. I. A modified OsO 4 fixative which selectively contrasts glycogen. J Ultrastruct Res. 1973 Jan;42(1):29–50. doi: 10.1016/s0022-5320(73)80004-8. [DOI] [PubMed] [Google Scholar]
- de Bruijn W. C., Koerten H. K., Cleton-Soeteman M. I., Blok-van Hoek C. J. Image analysis and X-ray microanalysis in cytochemistry. Scanning Microsc. 1987 Dec;1(4):1651–1667. [PubMed] [Google Scholar]