Abstract
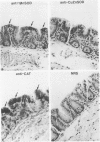
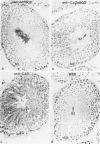
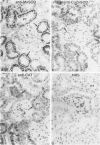
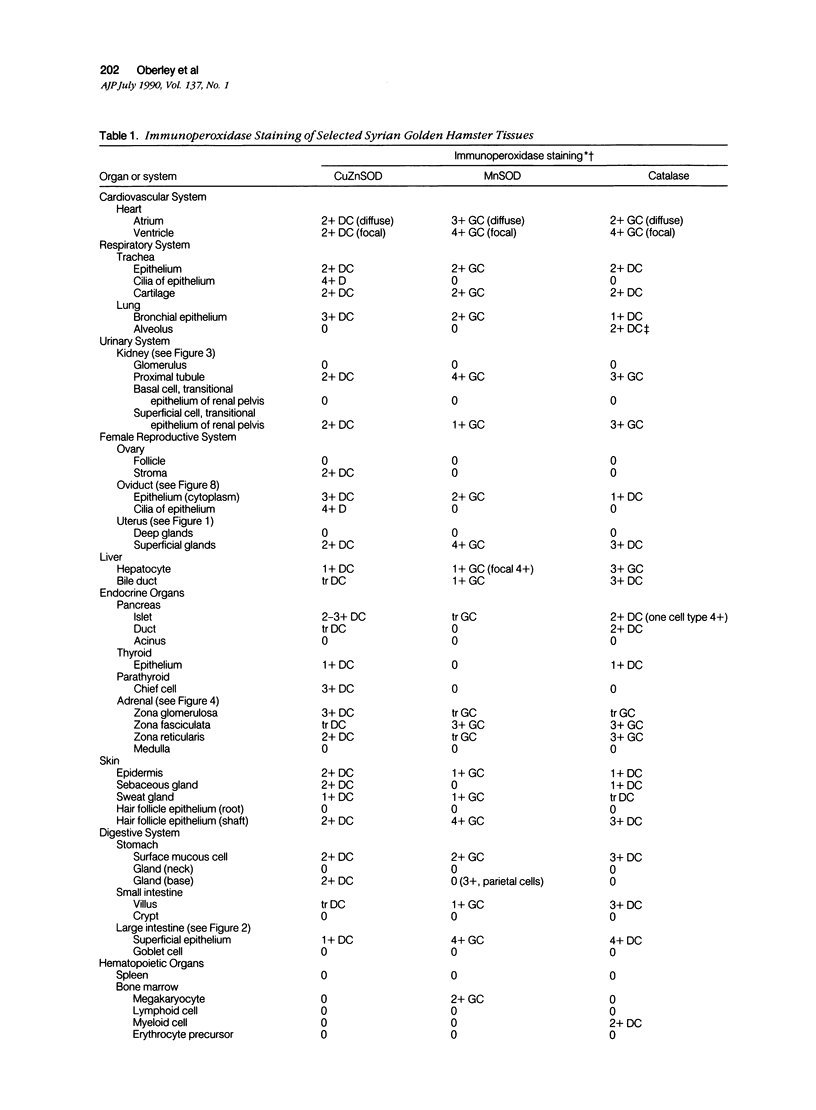
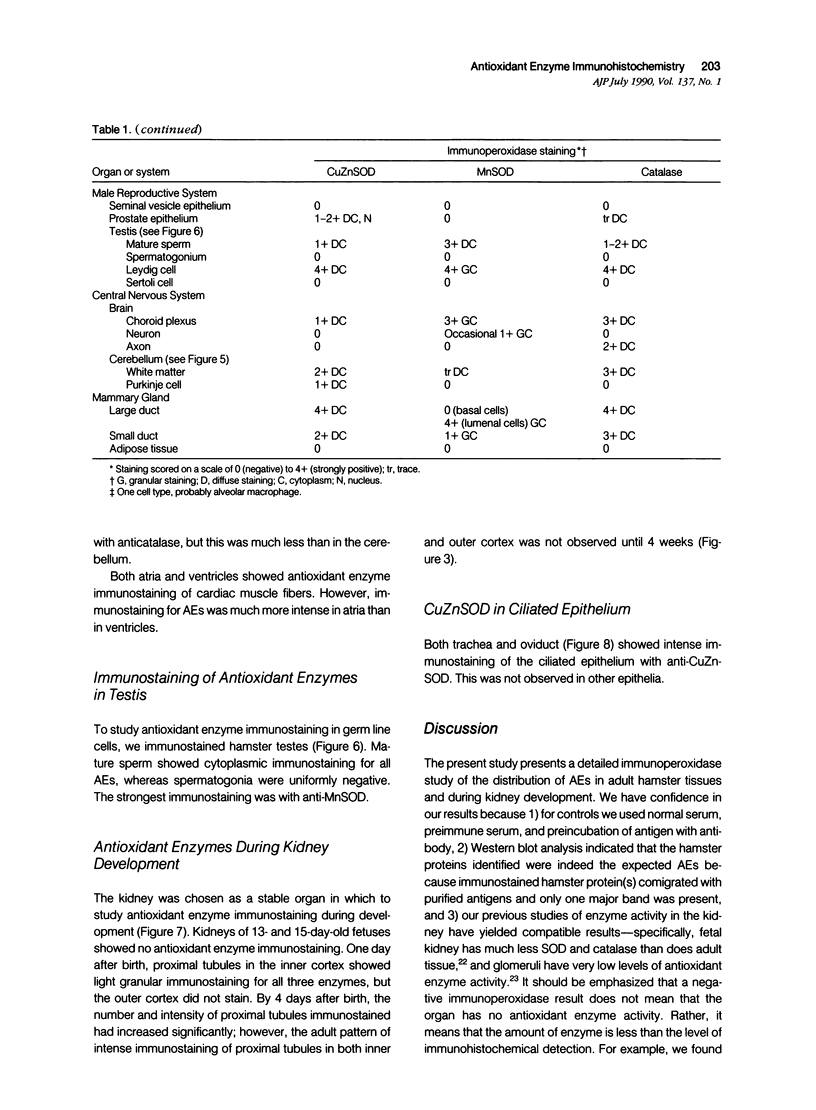
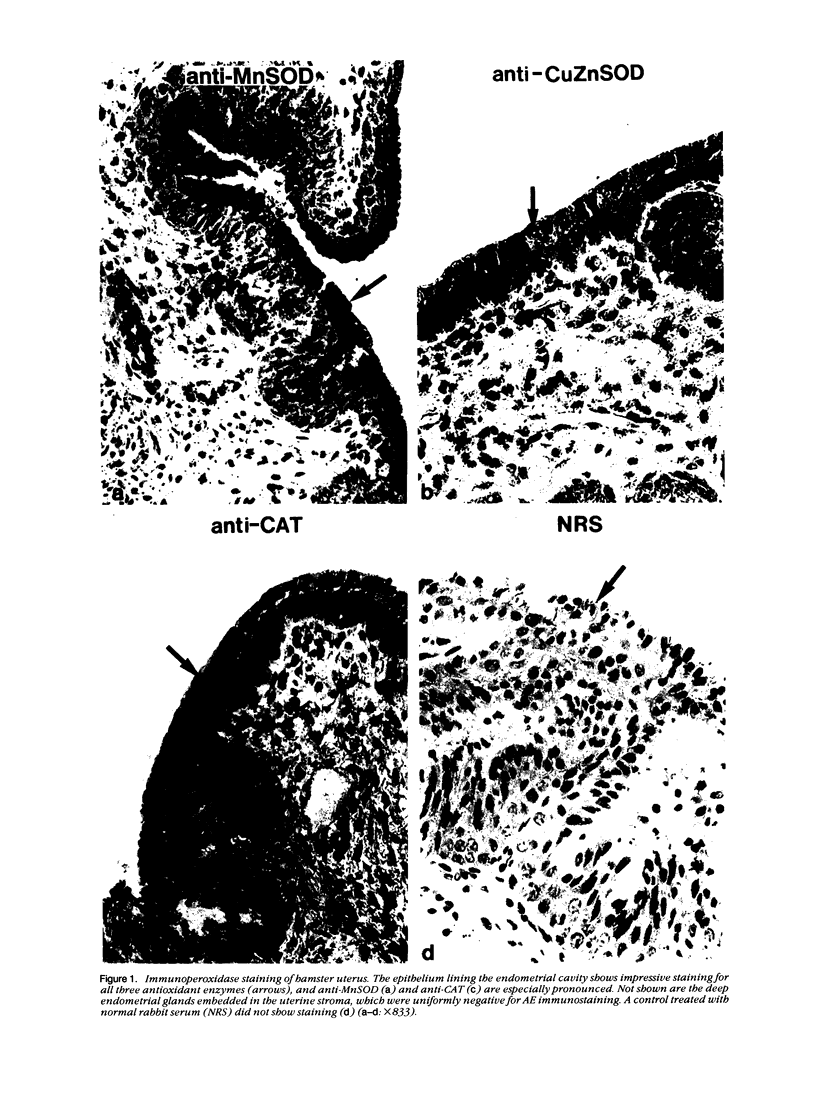
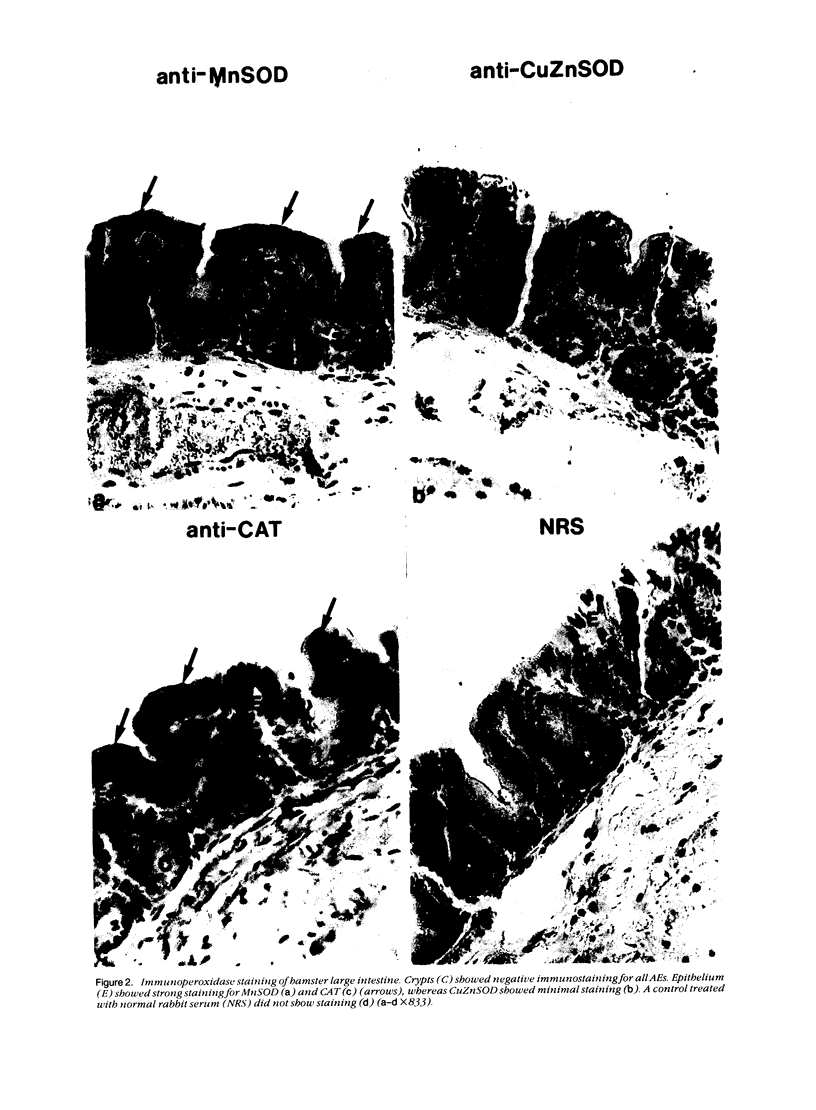
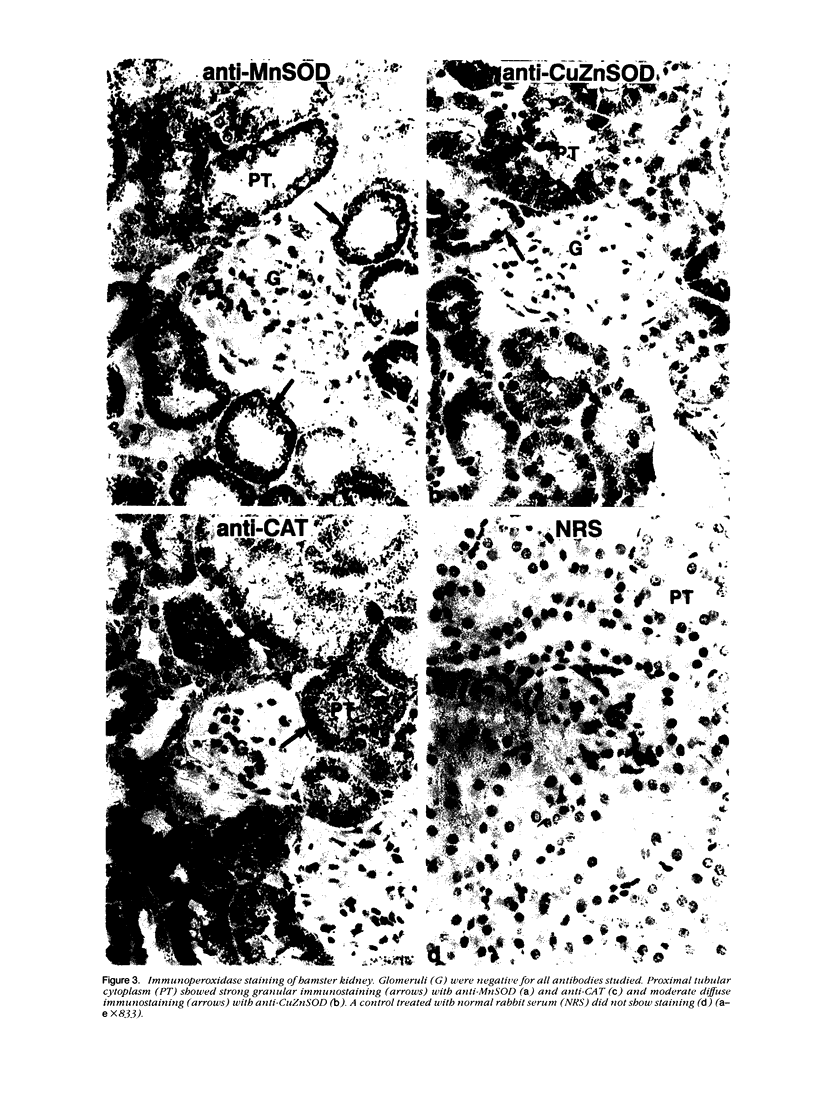
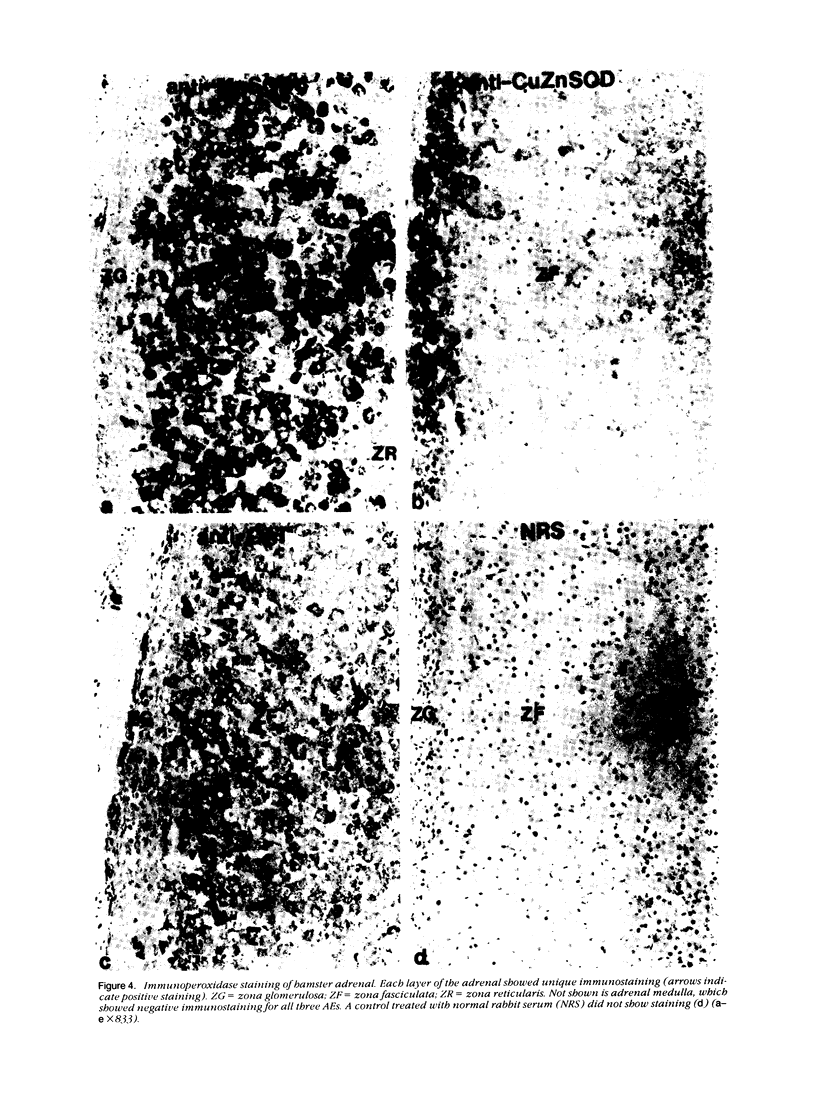
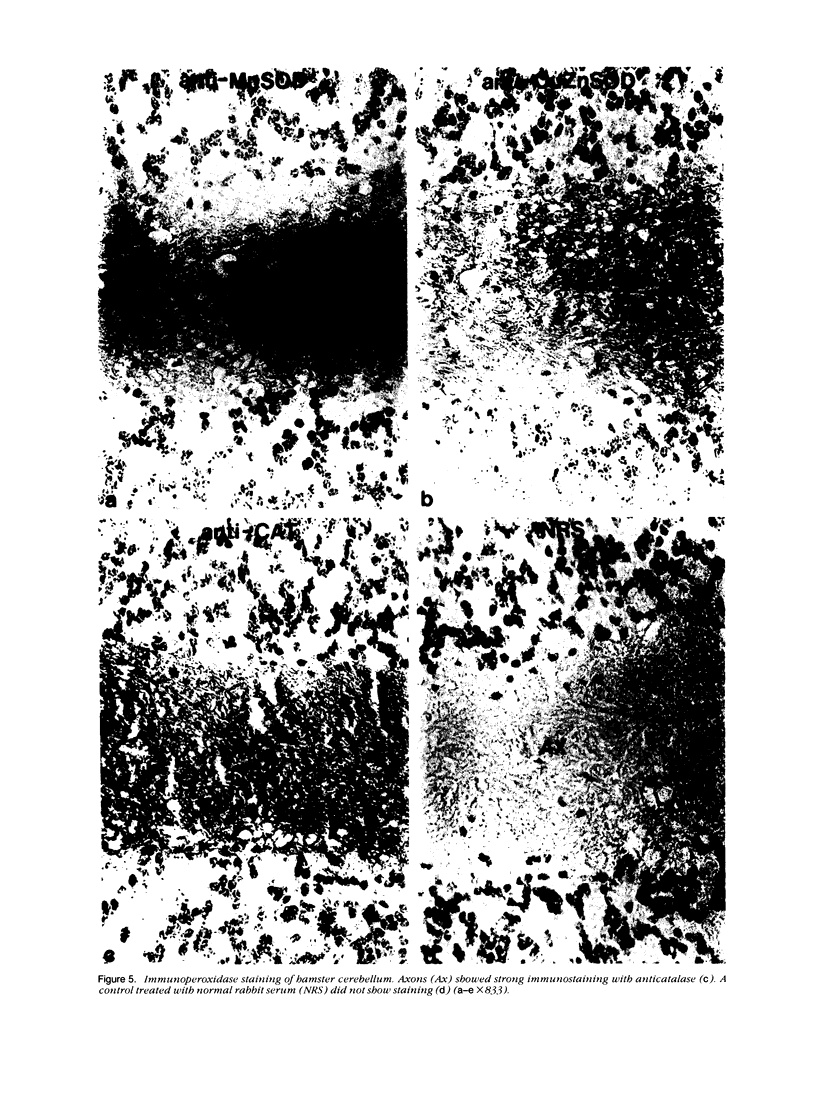
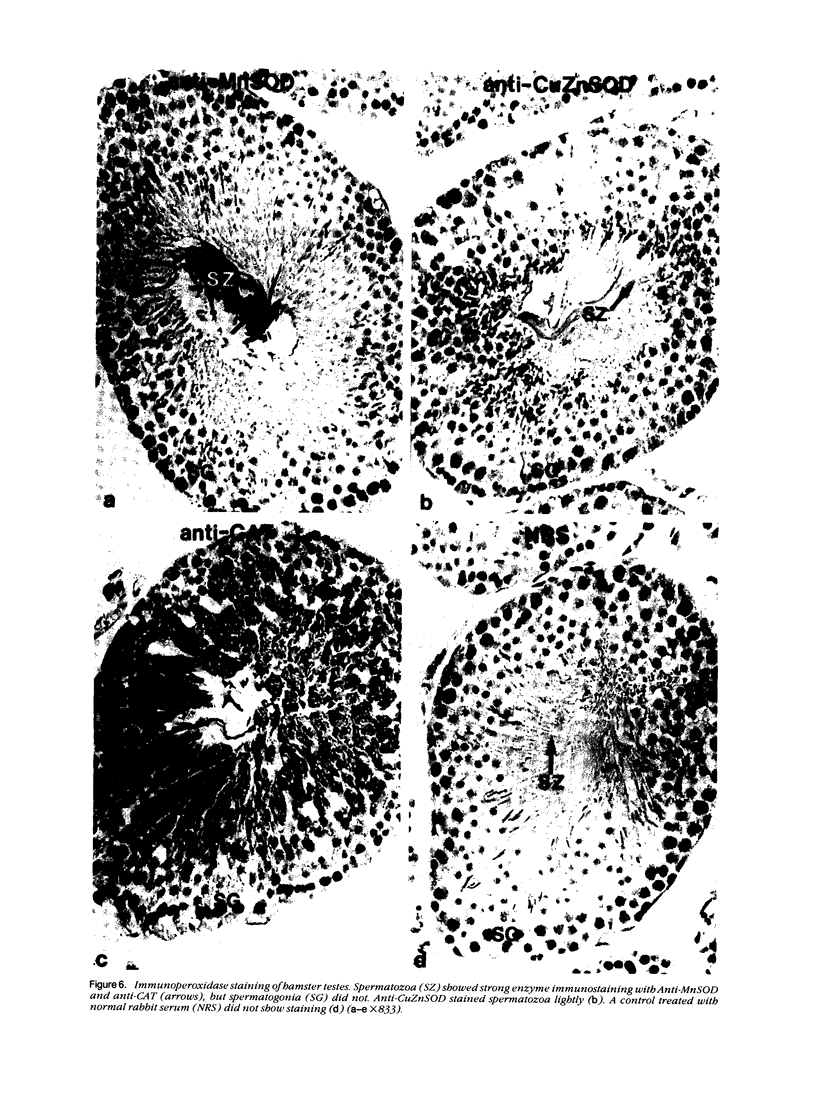
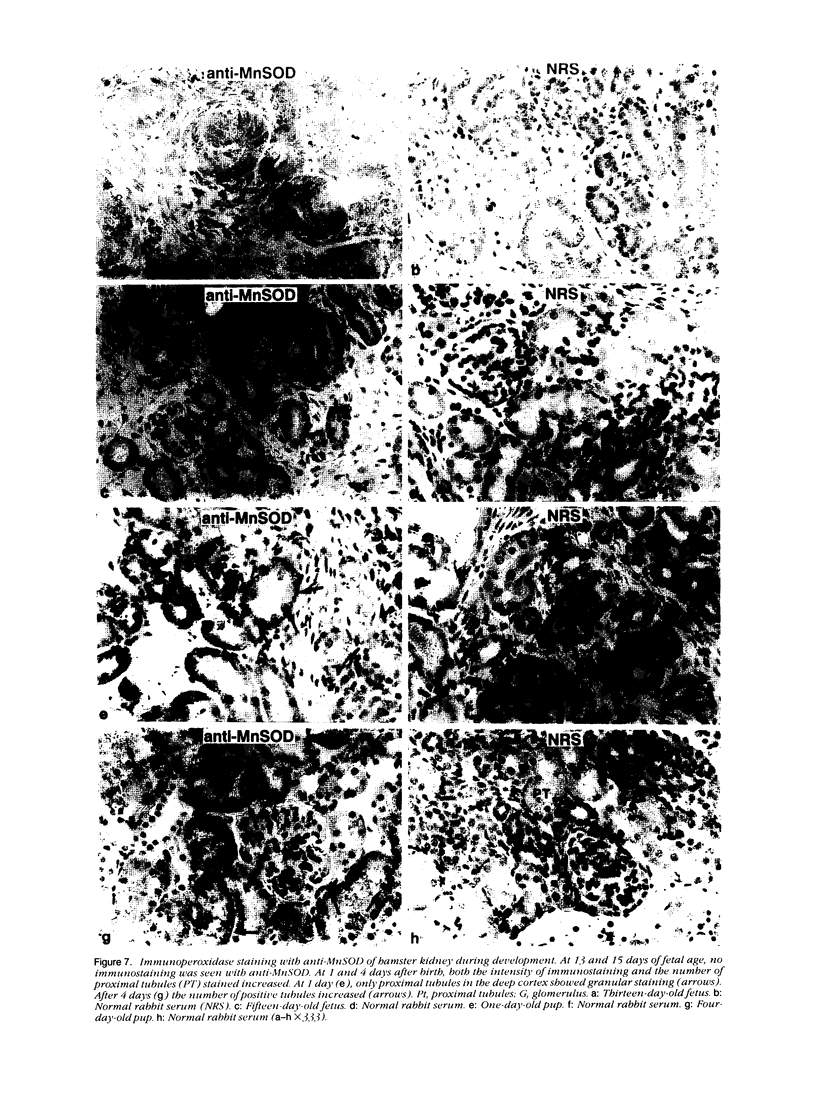
Tissues from adult Syrian hamsters were studied with immunoperoxidase techniques using polyclonal antibodies to three antioxidant enzymes (copper, zinc and manganese forms of superoxide dismutase, and catalase). Tissues from labile organs, in which cell renewal is prominent (uterus, intestine, and transitional epithelium of the urinary tract), showed strong antioxidant enzyme immunostaining in differentiated cells but not in stem cells. In stable organs, in which cell renewal occurs at a high rate only in response to injury (kidney and adrenal), each cell type showed a specific pattern of antioxidant enzyme immunostaining. In permanent organs (brain and heart), antioxidant enzymes were regionally specific markers. Axons of the cerebellum showed more intense antioxidant enzyme staining than those of the cerebral cortex; in the heart, atria stained more intensely than ventricles. Germ cells of the testis resembled cell renewal systems in their antioxidant enzyme-immunostaining pattern: spermatogonia were negative, whereas spermatozoa were strongly positive. The tubules of the kidney showed no antioxidant enzyme immunostaining until after birth. Our results suggest that there is a prominent role for antioxidant enzymes in cell differentiation during development and cell renewal.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Balin A. K. Oxidative influence on development and differentiation: an overview of a free radical theory of development. Free Radic Biol Med. 1989;6(6):631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- Armato U., Andreis P. G., Romano F. Exogenous Cu,Zn-superoxide dismutase suppresses the stimulation of neonatal rat hepatocytes' growth by tumor promoters. Carcinogenesis. 1984 Dec;5(12):1547–1555. doi: 10.1093/carcin/5.12.1547. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Benzi G., Marzatico F., Pastoris O., Villa R. F. Relationship between aging, drug treatment and the cerebral enzymatic antioxidant system. Exp Gerontol. 1989;24(2):137–148. doi: 10.1016/0531-5565(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. Superoxide radical and superoxide dismutases: threat and defense. Acta Physiol Scand Suppl. 1980;492:9–18. [PubMed] [Google Scholar]
- Carraro C., Pathak M. A. Characterization of superoxide dismutase from mammalian skin epidermis. J Invest Dermatol. 1988 Jan;90(1):31–36. doi: 10.1111/1523-1747.ep12462534. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Slot J. W., Geuze H. J., Crapo J. D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988 Dec;107(6 Pt 1):2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Kopan R., Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol. 1989 Nov;109(5):2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Grankvist K., Marklund S. L., Täljedal I. B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981 Nov 1;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid-Smith P. Analgesic nephropathy. Aust N Z J Med. 1988 May;18(3):251–254. doi: 10.1111/j.1445-5994.1988.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Kirkman H. N., Galiano S., Gaetani G. F. The function of catalase-bound NADPH. J Biol Chem. 1987 Jan 15;262(2):660–666. [PubMed] [Google Scholar]
- Loven D. P., Oberley L. W., Rousseau F. M., Stevens R. H. Superoxide dismutase activity in 1,2-dimethylhydrazine-induced rat colon adenocarcinoma. J Natl Cancer Inst. 1980 Aug;65(2):377–381. [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Westman N. G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982 May;42(5):1955–1961. [PubMed] [Google Scholar]
- Mason R. P., Chignell C. F. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981 Dec;33(4):189–211. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Buettner G. R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979 Apr;39(4):1141–1149. [PubMed] [Google Scholar]
- Oberley L. W., Ridnour L. A., Sierra-Rivera E., Oberley T. D., Guernsey D. L. Superoxide dismutase activities of differentiating clones from an immortal cell line. J Cell Physiol. 1989 Jan;138(1):50–60. doi: 10.1002/jcp.1041380109. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., St Clair D. K., Autor A. P., Oberley T. D. Increase in manganese superoxide dismutase activity in the mouse heart after X-irradiation. Arch Biochem Biophys. 1987 Apr;254(1):69–80. doi: 10.1016/0003-9861(87)90082-8. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Dwivedi R. S., Yeldandi A. V., Subbarao V., Tan X. D., Usman M. I., Thangada S., Nemali M. R., Kumar S., Scarpelli D. G. Role of periductal and ductular epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage. A change in the differentiation commitment. Am J Pathol. 1989 May;134(5):1069–1086. [PMC free article] [PubMed] [Google Scholar]
- Schallreuter K. U., Wood J. M. Free radical reduction in the human epidermis. Free Radic Biol Med. 1989;6(5):519–532. doi: 10.1016/0891-5849(89)90045-2. [DOI] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- Thaete L. G., Crouch R. K., Schulte B. A., Spicer S. S. The immunolocalization of copper-zinc superoxide dismutase in canine tissues. J Histochem Cytochem. 1983 Dec;31(12):1399–1406. doi: 10.1177/31.12.6355288. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Yang A. H., Oberley T. D., Oberley L. W., Ramanathan R. Effect of cell substrate on antioxidant enzyme activities in cultured renal glomerular epithelium. Am J Pathol. 1988 Mar;130(3):616–628. [PMC free article] [PubMed] [Google Scholar]