Abstract
Chronic aminonucleoside nephrosis is variably associated with tubulointerstitial damage, depending on the route and frequency of drug administration. Recently, different groups have shown this injurious tubulointerstitial process to be reversible, coinciding with the resolution of heavy proteinuria to normal values. The authors have previously shown that a single jugular intravenous administration of puromycin aminonucleoside (PA) to male Munich-Wistar rats produces a triphasic pattern of glomerular injury and proteinuria, which culminates in focal glomerulosclerosis 70 weeks after drug administration. The authors now report the later progression of the tubulointerstitial morphologic abnormalities associated with acute nephrosis (phase I), despite spontaneous resolution of glomerular injury during the intermediate period (phase II) in this model. Although treatment of rats with the angiotensin I converting enzyme inhibitor enalapril (50 mg/l drinking water) over the 70-week period did not affect the magnitude of proteinuria during the acute nephrotic phase, enalapril prevented the recurrence of proteinuria (phase III), as well as significantly reducing the severity of interstitial fibrosis, extent of tubular dilatation, and number of intratubular casts on semiquantitative scoring at the conclusion of the study. In addition, enalapril-treated rats had less low-molecular-weight protein excretion during the recurrent phase of proteinuria, suggesting a preservation of tubular functional capacity to reabsorb these proteins. In vitro cytotoxicity studies showed only the glomerular visceral epithelial cell to be sensitive to PA, in contrast with rat tubular epithelium and other cellular controls. Although the exact pathogenetic mechanism responsible for the development of the tubulointerstitial damage remains unknown, PA in vitro does not adversely affect rat tubular epithelium; there is however a clear correlation between the magnitude of recurrent proteinuria and the severity of tubulointerstitial morphologic abnormalities, as suggested by the beneficial effect of converting enzyme inhibition on both of these untoward processes.
Full text
PDF



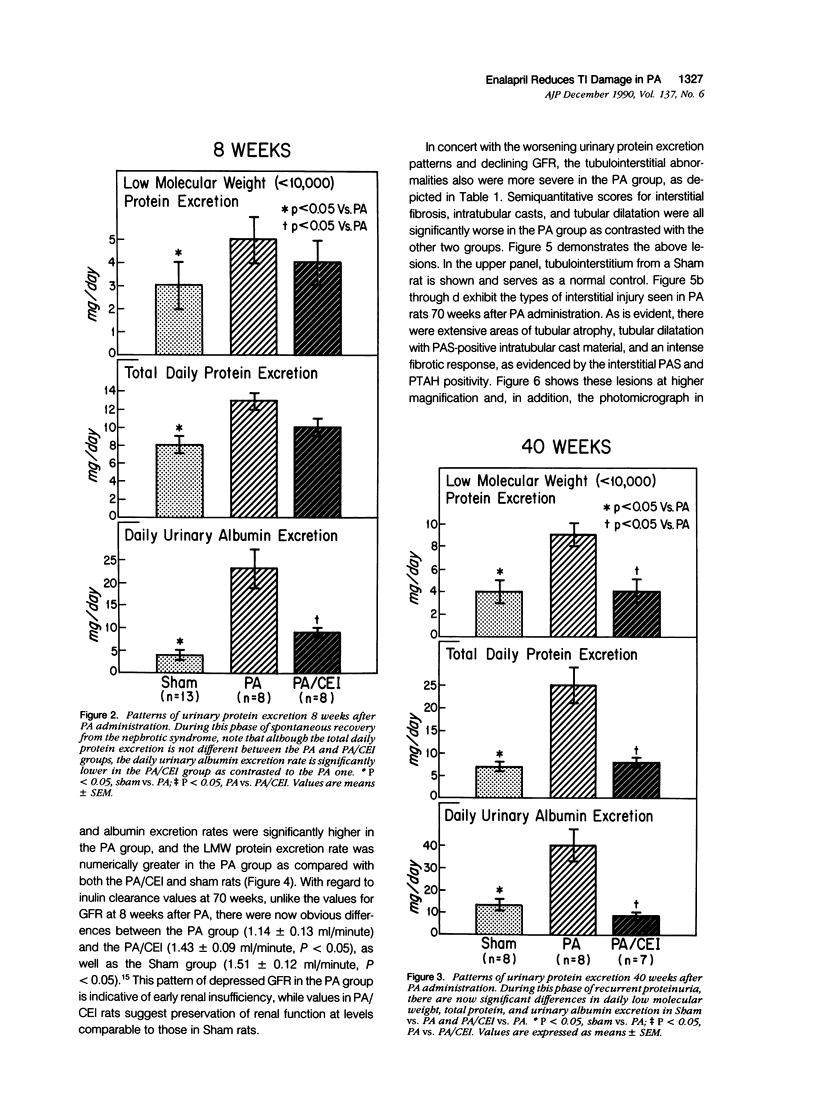
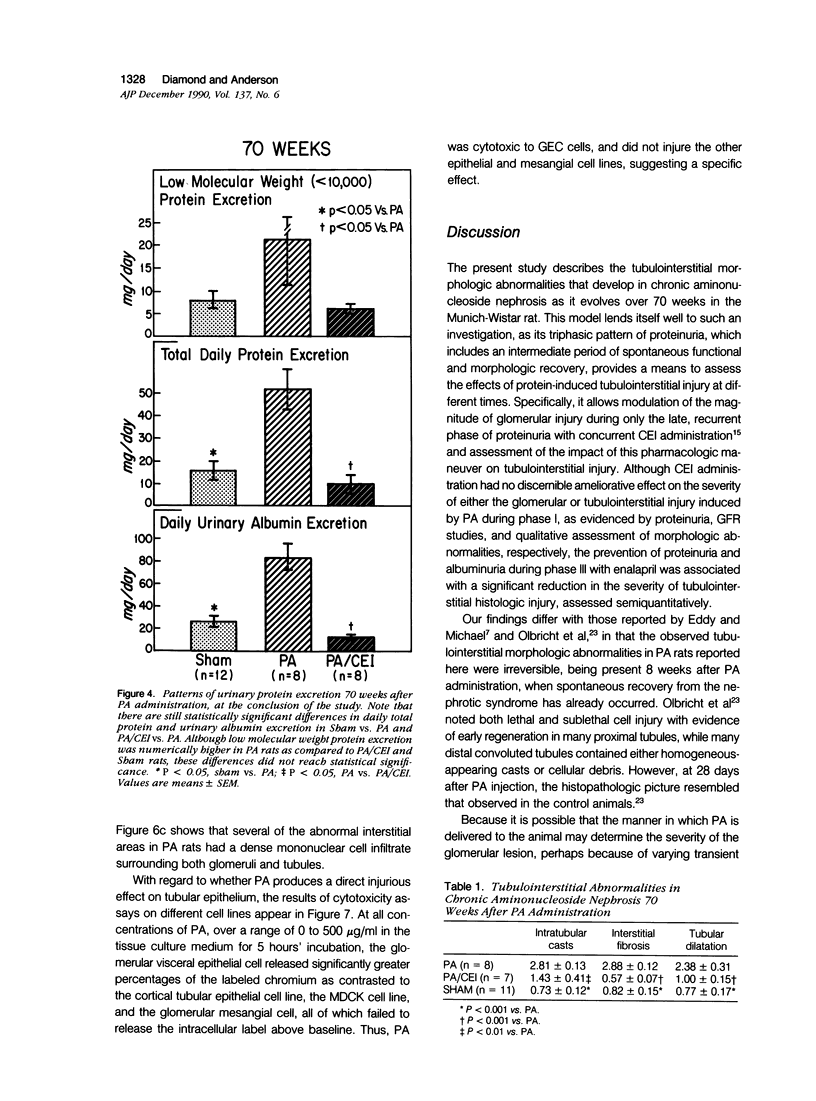

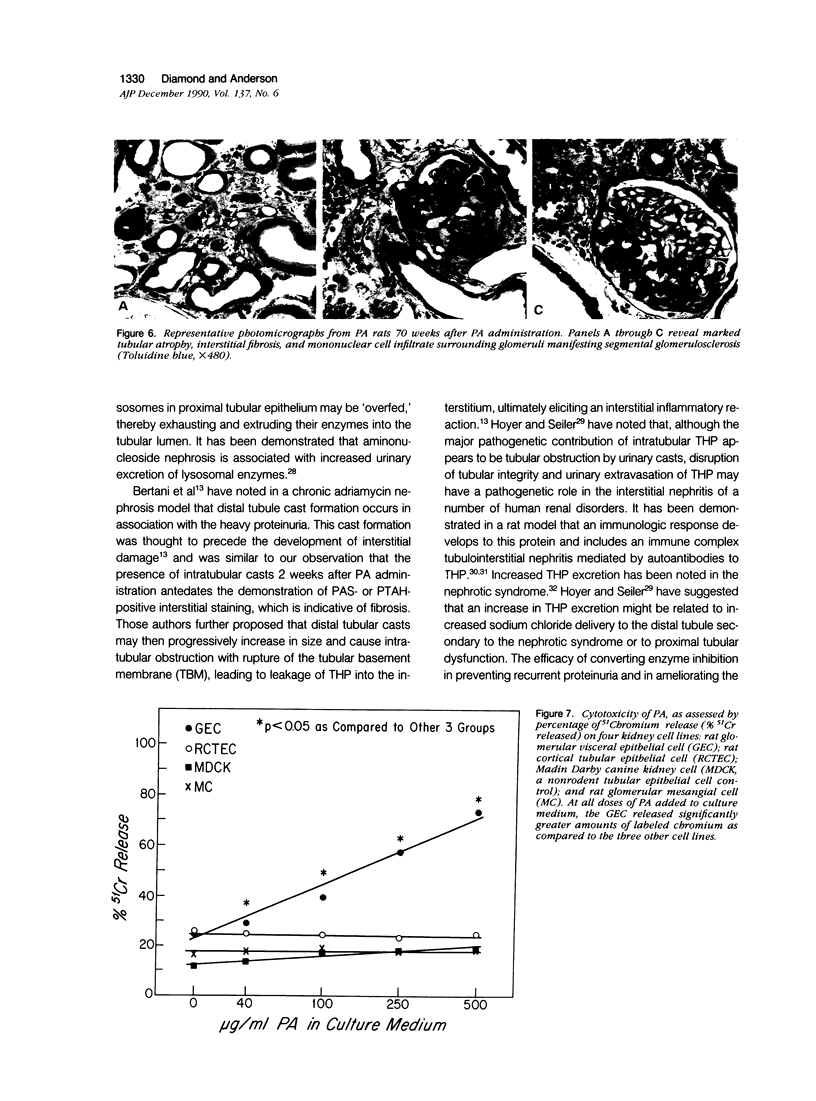


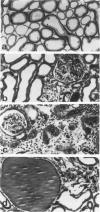
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus D., Mann R., Cohn D., Michaud L., Kelly C., Clayman M., Neilson E. G. Inhibitory role of dietary protein restriction on the development and expression of immune-mediated antitubular basement membrane-induced tubulointerstitial nephritis in rats. J Clin Invest. 1985 Sep;76(3):930–936. doi: 10.1172/JCI112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Diamond J. R., Karnovsky M. J., Brenner B. M. Mechanisms underlying transition from acute glomerular injury to late glomerular sclerosis in a rat model of nephrotic syndrome. J Clin Invest. 1988 Nov;82(5):1757–1768. doi: 10.1172/JCI113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Cutillo F., Zoja C., Broggini M., Remuzzi G. Tubulo-interstitial lesions mediate renal damage in adriamycin glomerulopathy. Kidney Int. 1986 Oct;30(4):488–496. doi: 10.1038/ki.1986.212. [DOI] [PubMed] [Google Scholar]
- Chandra M., Susin M., Teichberg S., McVicar M. Experimental focal segmental glomerulosclerosis: correlation with protein excretion, glomerular filtration rate, and renal plasma flow. Pediatr Res. 1984 Nov;18(11):1195–1201. doi: 10.1203/00006450-198411000-00029. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 1986 Mar;122(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Michael A. F. Acute tubulointerstitial nephritis associated with aminonucleoside nephrosis. Kidney Int. 1988 Jan;33(1):14–23. doi: 10.1038/ki.1988.3. [DOI] [PubMed] [Google Scholar]
- Exaire E., Pollak V. E., Pesce A. J., Ooi B. S. Albumin and -globulin in the nephron of the normal rat and following the injection of aminonucleoside. Nephron. 1972;9(1):42–54. doi: 10.1159/000180132. [DOI] [PubMed] [Google Scholar]
- FIEGELSON E. B., DRAKE J. W., RECANT L. Experimental aminonucleoside nephrosis in rats. J Lab Clin Med. 1957 Sep;50(3):437–446. [PubMed] [Google Scholar]
- Glasser R. J., Velosa J. A., Michael A. F. Experimental model of focal sclerosis. I. Relationship to protein excretion in aminonucleoside nephrosis. Lab Invest. 1977 May;36(5):519–526. [PubMed] [Google Scholar]
- Hoyer J. R., Seiler M. W. Pathophysiology of Tamm-Horsfall protein. Kidney Int. 1979 Sep;16(3):279–289. doi: 10.1038/ki.1979.130. [DOI] [PubMed] [Google Scholar]
- Kaysen G. A., Myers B. D., Couser W. G., Rabkin R., Felts J. M. Mechanisms and consequences of proteinuria. Lab Invest. 1986 May;54(5):479–498. [PubMed] [Google Scholar]
- Kleinknecht C., Laouari D., Gubler M. C., Gros F. Adverse effect of indomethacin in experimental chronic nephrosis. Int J Pediatr Nephrol. 1983 Jun;4(2):83–88. [PubMed] [Google Scholar]
- LANNIGAN R. The production of chronic renal disease in rats by a single intravenous injection of aminonucleoside of puromycin and the effect of low dosage continuous hydrocortisone. Br J Exp Pathol. 1963 Jun;44:326–333. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCKENZIE J. K., PATEL R., MCQUEEN E. G. THE EXCRETION RATE OF TAMM-HORSFALL URINARY MUCOPROTEIN IN NORMALS AND IN PATIENTS WITH RENAL DISEASE. Australas Ann Med. 1964 Feb;13:32–39. doi: 10.1111/imj.1964.13.1.32. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Melcion C., Smith J., Cattell V. Ferritin deposition in the glomerular deposits of focal glomerular sclerosis in the rat. J Pathol. 1984 Sep;144(1):45–55. doi: 10.1002/path.1711440106. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Bulger R. E., Striker G. E., Benditt E. P. Renal tubular effects of the aminonucleoside of puromycin. Lab Invest. 1972 May;26(5):558–565. [PubMed] [Google Scholar]
- Nath K. A., Hostetter M. K., Hostetter T. H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985 Aug;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Olbricht C. J., Cannon J. K., Tisher C. C. Cathepsin B and L in nephron segments of rats with puromycin aminonucleoside nephrosis. Kidney Int. 1987 Sep;32(3):354–361. doi: 10.1038/ki.1987.217. [DOI] [PubMed] [Google Scholar]
- Paigen K., Peterson J. Coordinacy of lysosomal enzyme excretion in human urine. J Clin Invest. 1978 Mar;61(3):751–762. doi: 10.1172/JCI108989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. G., Ellis B. G. Urinary enzyme excretion in aminonucleoside nephrosis in rats. Chem Biol Interact. 1976 Jun;13(3-4):353–358. doi: 10.1016/0009-2797(76)90088-0. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Tsuruda H., Okuda S., Onoyama K., Oh Y., Fujishima M. Effect of blood pressure on the progress of renal deterioration in rats with renal mass reduction. J Lab Clin Med. 1986 Jan;107(1):43–50. [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]