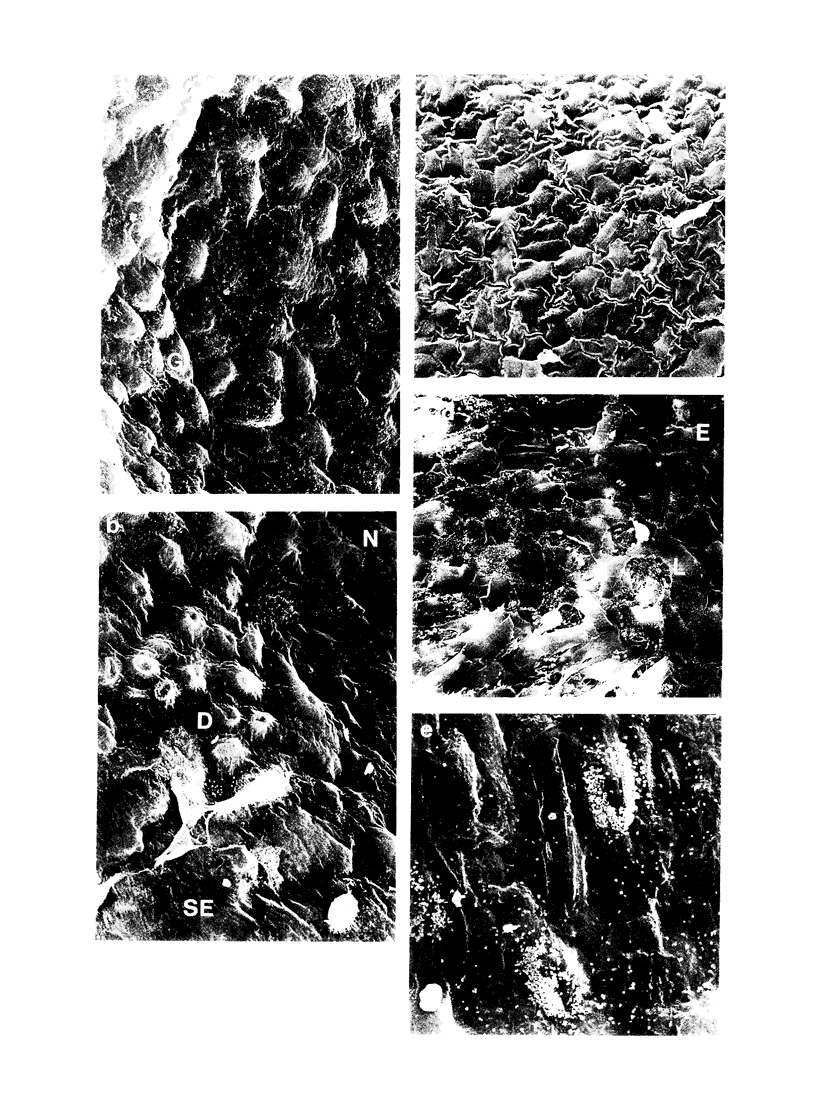
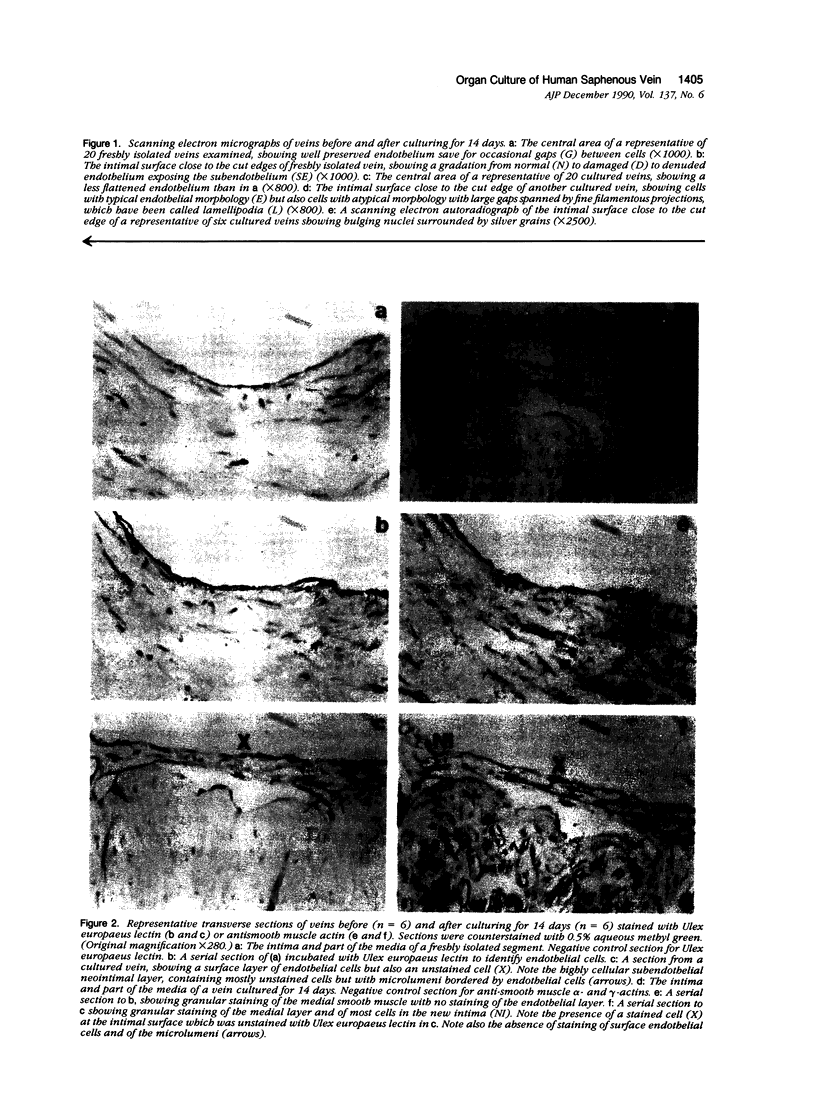
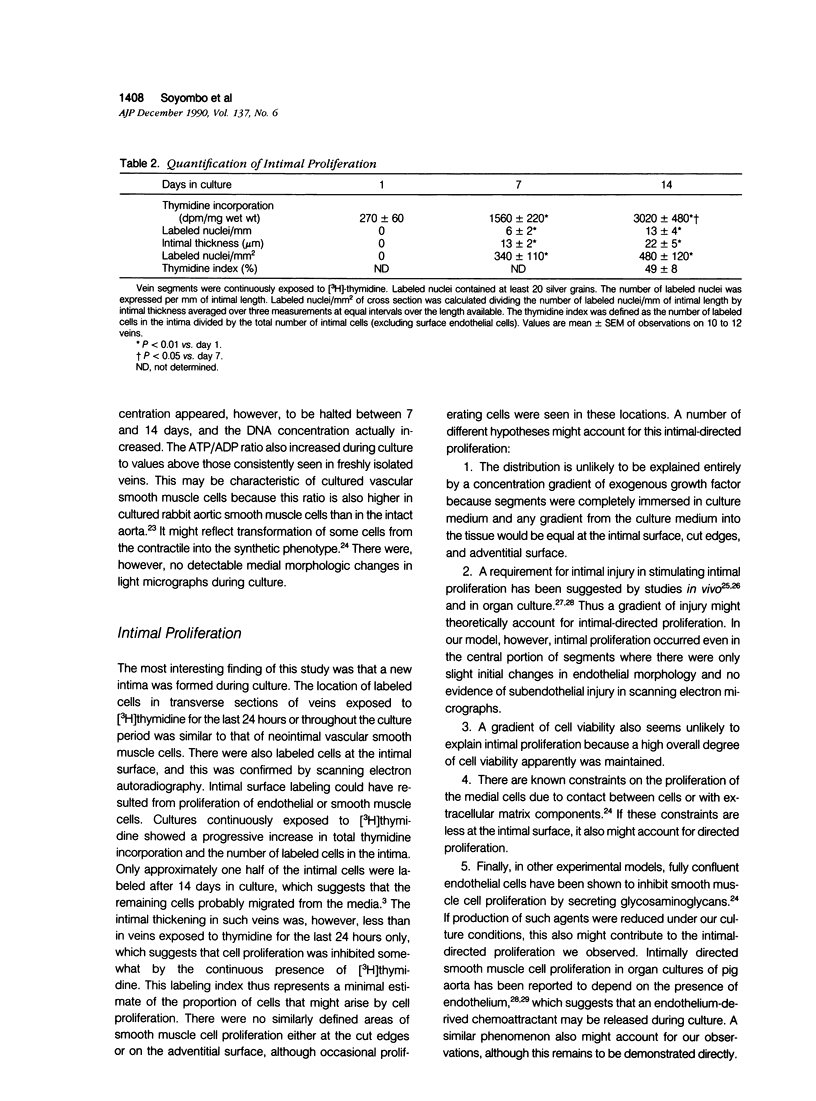
Abstract
This study investigated whether intimal proliferation, the characteristic feature of the response of human saphenous vein to arterial implantation, also occurs in organ culture. Vein segments were maintained for 14 days in medium supplemented with 30% fetal bovine serum. Tissue viability (measured by adenosine triphosphate [ATP] concentration) decreased only 20% from 280 +/- 20 to 220 +/- 20 nmol/g wet weight. In veins prepared for culturing, endothelial loss (approximately 20%) was confined to near the cut edges. Cultured veins retained an endothelial layer in the initially undamaged areas, while the initially injured areas became covered by a mixture of endothelial and vascular smooth muscle cells. Autoradiography in conjunction with scanning electron microscopy showed the presence of proliferating cells on the intimal surface. Transverse sections of cultured veins showed the development of a new intima containing vascular smooth muscle cells identified by immunocytochemistry with anti-alpha-actin. There were also endothelial cells identified with Ulex europaeus lectin arranged in capillarylike structures. Pulse or continuous labeling of cultures with [3H]thymidine showed that proliferating cells were confined to the new intima and suggested that the smooth muscle cells in this layer arose from both immigration and proliferation. The results demonstrate that intimal proliferation occurs in organ culture of human saphenous veins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang A. H., Tachas G., Campbell J. H., Bateman J. F., Campbell G. R. Collagen synthesis by cultured rabbit aortic smooth-muscle cells. Alteration with phenotype. Biochem J. 1990 Jan 15;265(2):461–469. doi: 10.1042/bj2650461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini G. D., Breckenridge I. M., Butchart E. G., Armistead S. H., Middleton K. M., Henderson A. H., Newby A. C. Metabolic damage to human saphenous vein during preparation for coronary artery bypass grafting. Cardiovasc Res. 1985 Jun;19(6):326–334. doi: 10.1093/cvr/19.6.326. [DOI] [PubMed] [Google Scholar]
- Angelini G. D., Newby A. C. The future of saphenous vein as a coronary artery bypass conduit. Eur Heart J. 1989 Mar;10(3):273–280. doi: 10.1093/oxfordjournals.eurheartj.a059476. [DOI] [PubMed] [Google Scholar]
- Angelini G. D., Passani S. L., Breckenridge I. M., Newby A. C. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987 Dec;21(12):902–907. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Campbell G. R. Endothelial cell influences on vascular smooth muscle phenotype. Annu Rev Physiol. 1986;48:295–306. doi: 10.1146/annurev.ph.48.030186.001455. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Dilley R. J., McGeachie J. K., Prendergast F. J. A review of the histologic changes in vein-to-artery grafts, with particular reference to intimal hyperplasia. Arch Surg. 1988 Jun;123(6):691–696. doi: 10.1001/archsurg.1988.01400300033004. [DOI] [PubMed] [Google Scholar]
- Feder J., Marasa J. C., Olander J. V. The formation of capillary-like tubes by calf aortic endothelial cells grown in vitro. J Cell Physiol. 1983 Jul;116(1):1–6. doi: 10.1002/jcp.1041160102. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Kraft T. The induction of smooth muscle cell proliferation in vitro using an organ culture system. Int Angiol. 1987 Jan-Mar;6(1):65–72. [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Fuchs J. C., Mitchener J. S., 3rd, Hagen P. O. Postoperative changes in autologous vein grafts. Ann Surg. 1978 Jul;188(1):1–15. doi: 10.1097/00000658-197807000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V., Chesebro J. J. Aortocoronary artery vein-graft disease: experimental and clinical approach for the understanding of the role of platelets and platelet inhibitors. Circulation. 1985 Dec;72(6 Pt 2):V65–V70. [PubMed] [Google Scholar]
- Gotlieb A. I., Boden P. Porcine aortic organ culture: a model to study the cellular response to vascular injury. In Vitro. 1984 Jul;20(7):535–542. doi: 10.1007/BF02639769. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Wong M. K., Boden P., Fone A. C. The role of the cytoskeleton in endothelial repair. Scanning Microsc. 1987 Dec;1(4):1715–1726. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Grondin C. M. Late results of coronary artery grafting: is there a flag on the field? J Thorac Cardiovasc Surg. 1984 Feb;87(2):161–166. [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Koo E. W., Gotlieb A. I. Endothelial stimulation of intimal cell proliferation in a porcine aortic organ culture. Am J Pathol. 1989 Mar;134(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Lytle B. W., Loop F. D., Cosgrove D. M., Ratliff N. B., Easley K., Taylor P. C. Long-term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J Thorac Cardiovasc Surg. 1985 Feb;89(2):248–258. [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson D. C., Bowyer D. E. Endothelial injury and healing in vitro. Studies using an organ culture system. Am J Pathol. 1985 May;119(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Reidy M. A., Silver M. Endothelial regeneration. VII. Lack of intimal proliferation after defined injury to rat aorta. Am J Pathol. 1985 Feb;118(2):173–177. [PMC free article] [PubMed] [Google Scholar]
- Roberts A. J., Hay D. A., Mehta J. L., Mehta P., Roy L., Faro R. S., Knauf D. G., Alexander J. A. Biochemical and ultrastructural integrity of the saphenous vein conduit during coronary artery bypass grafting. Preliminary results of the effect of papaverine. J Thorac Cardiovasc Surg. 1984 Jul;88(1):39–48. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Southgate K., Newby A. C. Serum-induced proliferation of rabbit aortic smooth muscle cells from the contractile state is inhibited by 8-Br-cAMP but not 8-Br-cGMP. Atherosclerosis. 1990 May;82(1-2):113–123. doi: 10.1016/0021-9150(90)90150-h. [DOI] [PubMed] [Google Scholar]
- Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988 Aug 11;319(6):332–337. doi: 10.1056/NEJM198808113190603. [DOI] [PubMed] [Google Scholar]
- Zwolak R. M., Adams M. C., Clowes A. W. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987 Jan;5(1):126–136. [PubMed] [Google Scholar]