Abstract
The evolution of senile plaques and the relationships among neuritic elements, extracellular deposits of the beta-amyloid protein (beta/A4), and vascular beta/A4 are poorly understood. Immunocytochemical methods were used to examine fixed-frozen prefrontal cortices of 14 rhesus monkeys (Macaca mulatta) (14 to 37 years of age) for the presence of abnormal fibers/neurites, alpha 1-antichymotrypsin (alpha-ACT), and beta/A4. Age-associated alterations included abnormal fibers/neurites, presence of beta/A4, and association of alpha-ACT with beta/A4 in plaques and blood vessels. Vascular amyloid was present only in the oldest monkeys. The topographic distribution of abnormal fibers/neurites was mapped with acetylcholinesterase (AChE) histochemistry, and deposits of amyloid were visualized with immunocytochemistry for beta/A4. beta/A4 often was associated with neurites, but many neurites lacked demonstrable beta/A4. Thus in aged monkeys, abnormal neurites may provide one type of focus for the accumulation of the amyloid precursor, which is subsequently abnormally processed to form beta/A4. Our data in rhesus monkeys suggest that fiber and neuritic abnormalities increase with age and that they may precede the majority of beta/A4 deposits; the initial stages of neurite formation and parenchymal amyloid deposits may be independent of the appearance of vascular amyloid; and these processes may be synergistic with advanced age.
Full text
PDF


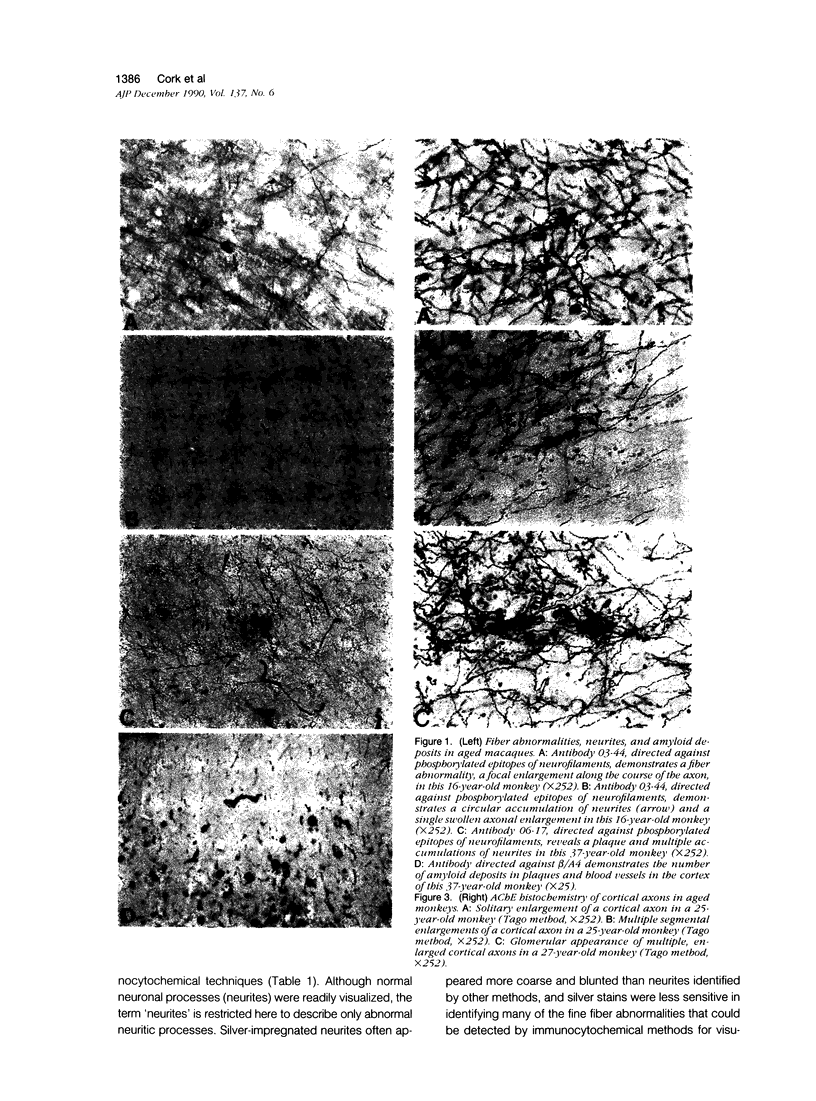


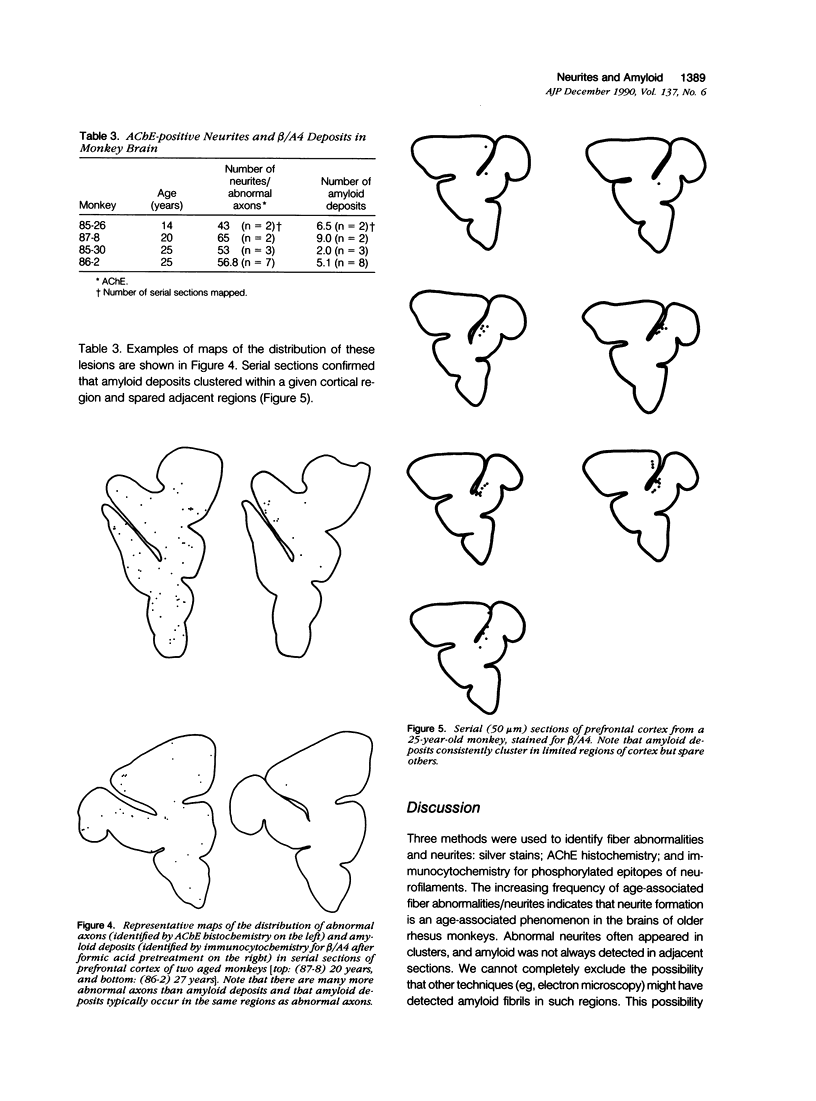



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Abraham C. R., Selkoe D. J., Potter H., Price D. L., Cork L. C. Alpha 1-antichymotrypsin is present together with the beta-protein in monkey brain amyloid deposits. Neuroscience. 1989;32(3):715–720. doi: 10.1016/0306-4522(89)90292-3. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Frangione B. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest. 1988 Feb;58(2):122–132. [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Allsop D., Glenner G. G. Morphology and distribution of plaque and related deposits in the brains of Alzheimer's disease and control cases. An immunohistochemical study using amyloid beta-protein antibody. Lab Invest. 1989 Jan;60(1):113–122. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Kitt C. A., Price D. L., Struble R. G., Cork L. C., Wainer B. H., Becher M. W., Mobley W. C. Evidence for cholinergic neurites in senile plaques. Science. 1984 Dec 21;226(4681):1443–1445. doi: 10.1126/science.6505701. [DOI] [PubMed] [Google Scholar]
- Kitt C. A., Struble R. G., Cork L. C., Mobley W. C., Walker L. C., Joh T. H., Price D. L. Catecholaminergic neurites in senile plaques in prefrontal cortex of aged nonhuman primates. Neuroscience. 1985 Nov;16(3):691–699. doi: 10.1016/0306-4522(85)90202-7. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Cork L. C., Unterbeck A., Bayney R. M., Price D. L. Differential expression of amyloid precursor protein mRNAs in cases of Alzheimer's disease and in aged nonhuman primates. Neuron. 1990 Jan;4(1):97–104. doi: 10.1016/0896-6273(90)90446-m. [DOI] [PubMed] [Google Scholar]
- Mandybur T. I. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986 Jan;45(1):79–90. [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmert M. R., Siedlak S. L., Podlisny M. B., Greenberg B., Shelton E. R., Chan H. W., Usiak M., Selkoe D. J., Perry G., Younkin S. G. Soluble derivatives of the beta amyloid protein precursor of Alzheimer's disease are labeled by antisera to the beta amyloid protein. Biochem Biophys Res Commun. 1989 Nov 30;165(1):182–188. doi: 10.1016/0006-291x(89)91052-8. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Presty S. K., Bachevalier J., Walker L. C., Struble R. G., Price D. L., Mishkin M., Cork L. C. Age differences in recognition memory of the rhesus monkey (Macaca mulatta). Neurobiol Aging. 1987 Sep-Oct;8(5):435–440. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Price D. L. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis N. K., Wisniewski H. M., Jenkins E. C., Devine-Gage E. A., Houck G. E., Yao X. L., Ramakrishna N., Wolfe G., Silverman W. P., Brown W. T. Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987 Feb 14;1(8529):384–385. doi: 10.1016/s0140-6736(87)91754-5. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Struble R. G., Cork L. C., Whitehouse P. J., Price D. L. Cholinergic innervation in neuritic plaques. Science. 1982 Apr 23;216(4544):413–415. doi: 10.1126/science.6803359. [DOI] [PubMed] [Google Scholar]
- Struble R. G., Kitt C. A., Walker L. C., Cork L. C., Price D. L. Somatostatinergic neurites in senile plaques of aged non-human primates. Brain Res. 1984 Dec 24;324(2):394–396. doi: 10.1016/0006-8993(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Struble R. G., Price D. L., Jr, Cork L. C., Price D. L. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985 Dec 30;361(1-2):267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- Tago H., Kimura H., Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986 Nov;34(11):1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Kitt C. A., Cork L. C., Struble R. G., Dellovade T. L., Price D. L. Multiple transmitter systems contribute neurites to individual senile plaques. J Neuropathol Exp Neurol. 1988 Mar;47(2):138–144. doi: 10.1097/00005072-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. C., Kitt C. A., Schwam E., Buckwald B., Garcia F., Sepinwall J., Price D. L. Senile plaques in aged squirrel monkeys. Neurobiol Aging. 1987 Jul-Aug;8(4):291–296. doi: 10.1016/0197-4580(87)90067-4. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Kitt C. A., Struble R. G., Schmechel D. E., Oertel W. H., Cork L. C., Price D. L. Glutamic acid decarboxylase-like immunoreactive neurites in senile plaques. Neurosci Lett. 1985 Aug 30;59(2):165–169. doi: 10.1016/0304-3940(85)90194-6. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Kitt C. A., Struble R. G., Wagster M. V., Price D. L., Cork L. C. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988 Sep-Dec;9(5-6):657–666. doi: 10.1016/s0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wen G. Y., Kim K. S. Comparison of four staining methods on the detection of neuritic plaques. Acta Neuropathol. 1989;78(1):22–27. doi: 10.1007/BF00687398. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Terry R. D. Morphology of the aging brain, human and animal. Prog Brain Res. 1973;40(0):167–186. doi: 10.1016/S0079-6123(08)60686-X. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M., Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989 Oct;135(4):593–597. [PMC free article] [PubMed] [Google Scholar]





