Abstract
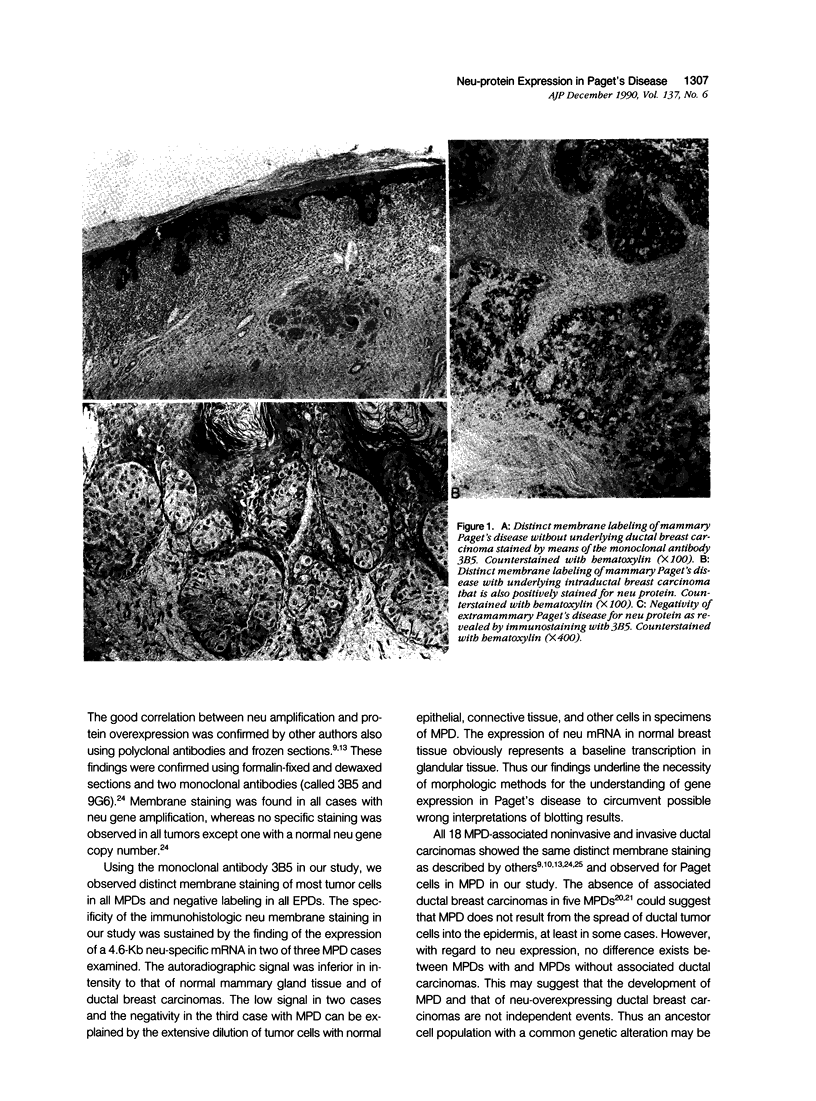
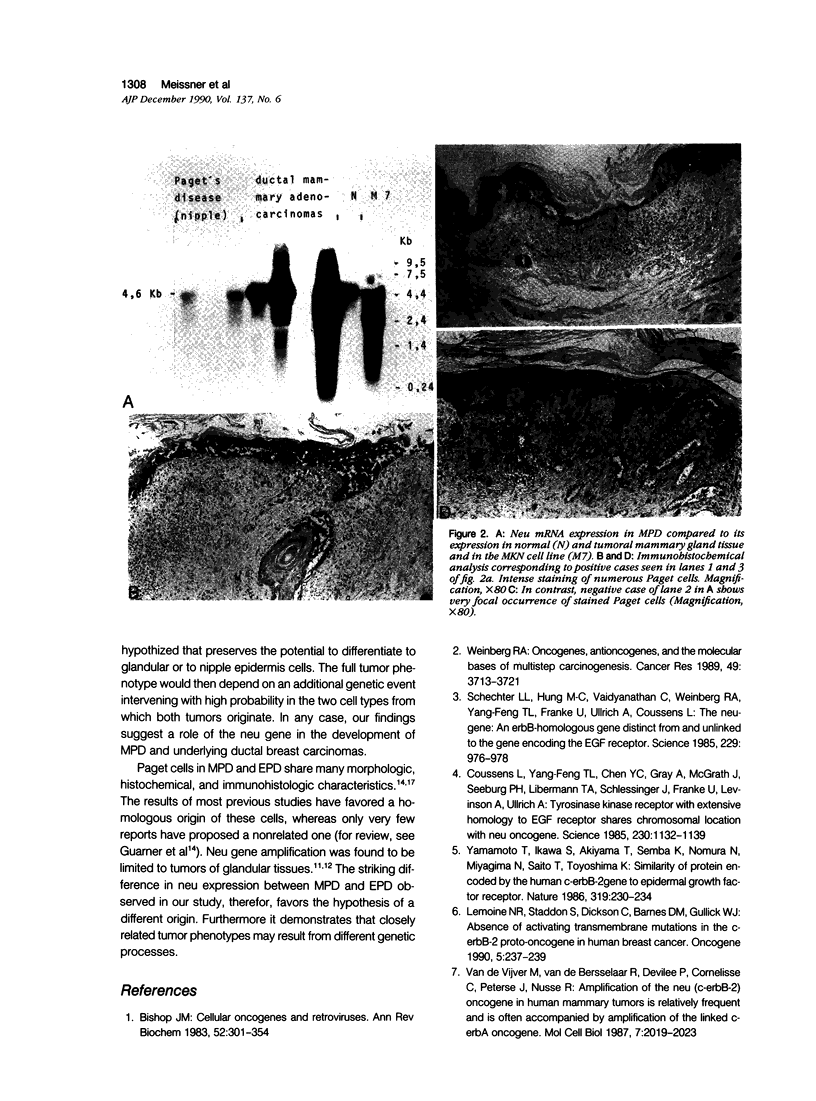
Correlation between neu/c-erbB-2/Her-2 gene amplification and overexpression of the neu gene product has been reported in tumors of glandular origin, especially ductal breast carcinomas. Formalin-fixed and dewaxed sections from 23 cases of mammary (MPD) and 9 cases of extramammary (EPD) Paget's disease were immunohistochemically stained by means of the monoclonal antibody 3B5 directed against an intracellular domain of the neu gene protein. All MPDs exhibited a distinct membrane staining of tumor cells independent of the presence of ductal breast carcinomas found in 18 cases. All these breast carcinomas also were positive for neu staining. In contrast to MPD, all EPDs were negative. Normal epidermis was always negative. Northern blot analysis sustained the immunohistologic findings in that the presence of neu mRNA could be demonstrated in two of three cases with MPD. Negativity in one case was due to dilution effects by nontumor cells. Our results suggest that Paget cells of mammary and extramammary localization, although very similar phenotypically, derive from different genetic accidents. Furthermore neu positivity in all MPDs and all underlying ductal carcinomas suggests common genetic alterations for both tumors. However the finding of five neu protein-positive MPDs without associated ductal breast carcinomas may suggest a somewhat different transformation process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M. S., Locher G. W., Saurer S., Gullick W. J., Waterfield M. D., Groner B., Hynes N. E. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988 Mar 1;48(5):1238–1243. [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T. L., Liao Y. C., Chen E., Gray A., McGrath J., Seeburg P. H., Libermann T. A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985 Dec 6;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Guarner J., Cohen C., DeRose P. B. Histogenesis of extramammary and mammary Paget cells. An immunohistochemical study. Am J Dermatopathol. 1989 Aug;11(4):313–318. doi: 10.1097/00000372-198908000-00004. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Jr, Austin C., Ackerman A. B. Extramammary Paget's disease. A critical reexamination. Am J Dermatopathol. 1979 Summer;1(2):101–132. doi: 10.1097/00000372-197900120-00002. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Jr Mammary Paget's disease without underlying carcinoma. Am J Dermatopathol. 1985 Aug;7(4):361–365. doi: 10.1097/00000372-198508000-00009. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Staddon S., Dickson C., Barnes D. M., Gullick W. J. Absence of activating transmembrane mutations in the c-erbB-2 proto-oncogene in human breast cancer. Oncogene. 1990 Feb;5(2):237–239. [PubMed] [Google Scholar]
- Mariani-Costantini R., Andreola S., Rilke F. Tumour-associated antigens in mammary and extramammary Paget's disease. Virchows Arch A Pathol Anat Histopathol. 1985;405(3):333–340. doi: 10.1007/BF00710069. [DOI] [PubMed] [Google Scholar]
- Marx D., Schauer A., Reiche C., May A., Ummenhofer L., Reles A., Rauschecker H., Sauer R., Schumacher M. c-erbB2 expression in correlation to other biological parameters of breast cancer. J Cancer Res Clin Oncol. 1990;116(1):15–20. doi: 10.1007/BF01612634. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Lucas D. O., McDaniel K. M., Clark V. A., Schmalzel G. M. Paget's cells. New evidence linking mammary and extramammary Paget cells to a common cell phenotype. Am J Clin Pathol. 1985 Apr;83(4):431–438. doi: 10.1093/ajcp/83.4.431. [DOI] [PubMed] [Google Scholar]
- Ordóez N. G., Awalt H., Mackay B. Mammary and extramammary Paget's disease. An immunocytochemical and ultrastructural study. Cancer. 1987 Mar 15;59(6):1173–1183. doi: 10.1002/1097-0142(19870315)59:6<1173::aid-cncr2820590624>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Hung M. C., Vaidyanathan L., Weinberg R. A., Yang-Feng T. L., Francke U., Ullrich A., Coussens L. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 1985 Sep 6;229(4717):976–978. doi: 10.1126/science.2992090. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Swallow J. E., Brammar W. J., Whittaker J. L., Walker R. A. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1(4):423–430. [PubMed] [Google Scholar]
- Venter D. J., Tuzi N. L., Kumar S., Gullick W. J. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet. 1987 Jul 11;2(8550):69–72. doi: 10.1016/s0140-6736(87)92736-x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- Yokota J., Yamamoto T., Toyoshima K., Terada M., Sugimura T., Battifora H., Cline M. J. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986 Apr 5;1(8484):765–767. doi: 10.1016/s0140-6736(86)91782-4. [DOI] [PubMed] [Google Scholar]
- Zhou D., Battifora H., Yokota J., Yamamoto T., Cline M. J. Association of multiple copies of the c-erbB-2 oncogene with spread of breast cancer. Cancer Res. 1987 Nov 15;47(22):6123–6125. [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- van de Vijver M., van de Bersselaar R., Devilee P., Cornelisse C., Peterse J., Nusse R. Amplification of the neu (c-erbB-2) oncogene in human mammmary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol. 1987 May;7(5):2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]