Abstract
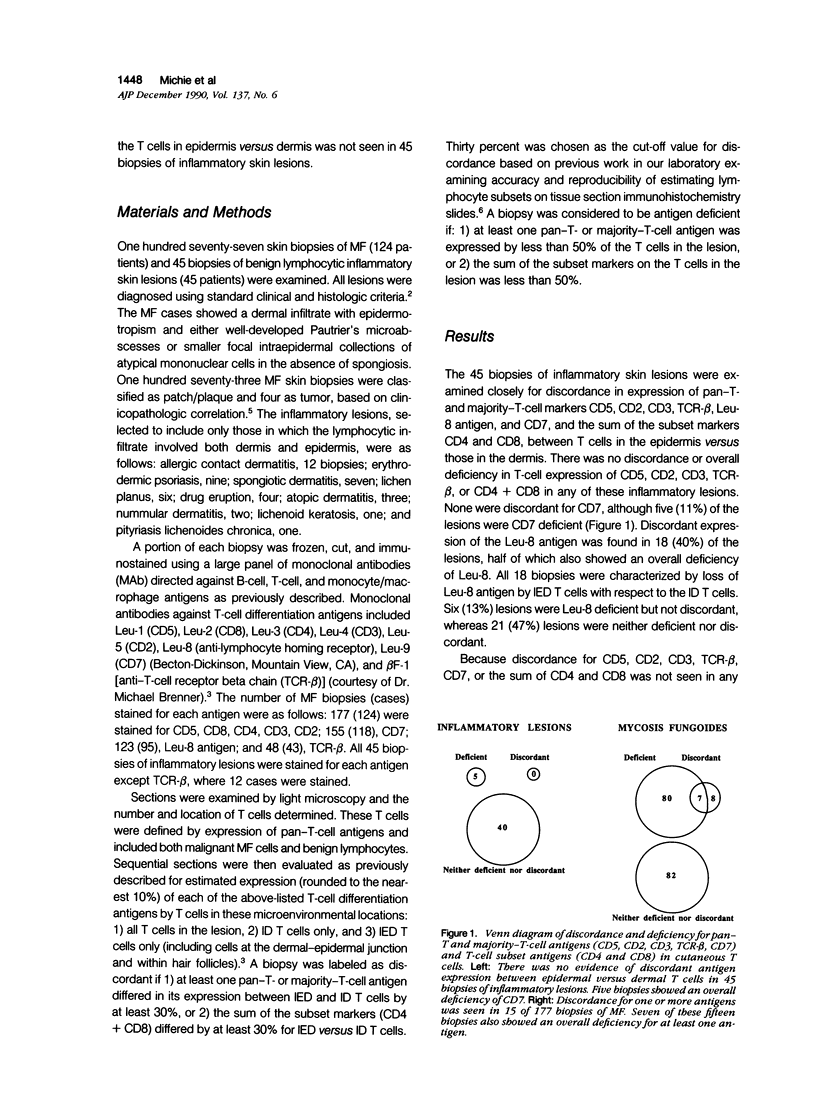
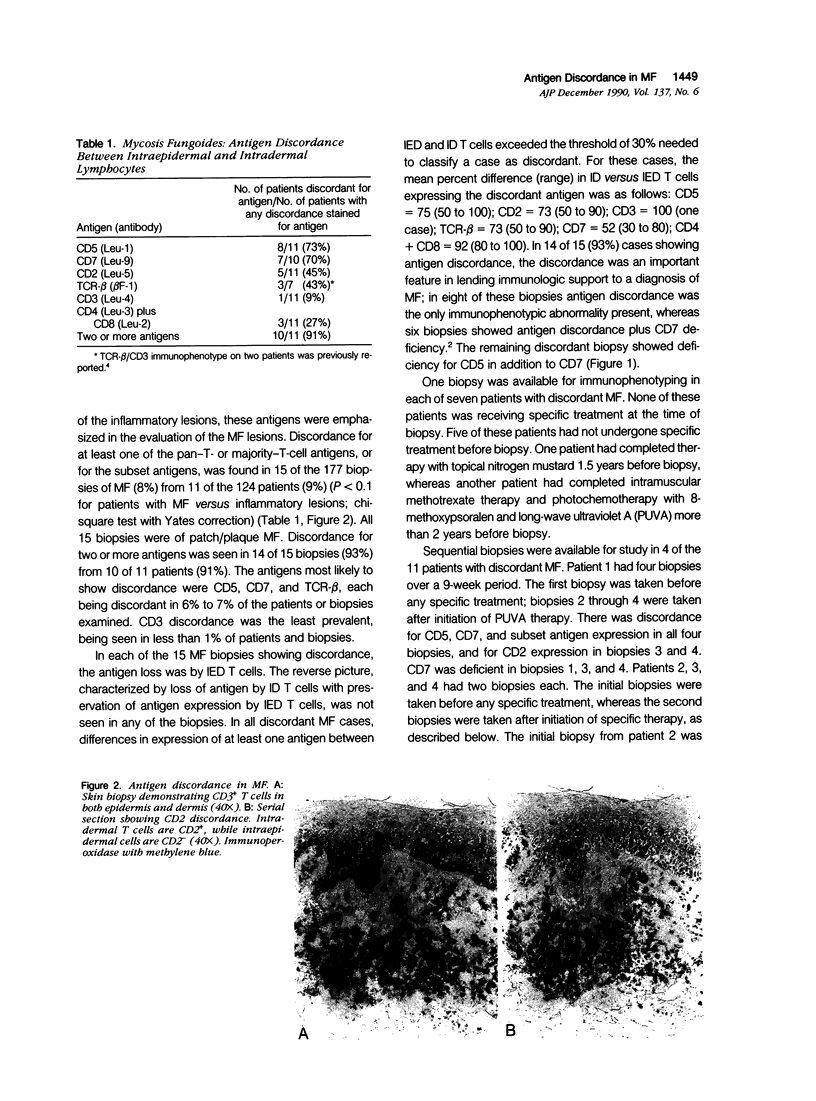
Using immunohistochemical methods, the authors studied the expression of pan-T- and majority-T-cell antigens (CD5, CD2, CD3, TCR-beta, CD7) and T-cell subset antigens (CD4, CD8) in cutaneous T cells in mycosis fungoides (MF) (177 biopsies from 124 patients) and a variety of inflammatory lesions (45 biopsies from 45 patients). The authors detected the absence of pan-T- or majority-T-cell antigens, or of both T-cell subset antigens, from T cells in the epidermis but not the dermis in 15 MF biopsies (8%) from 11 MF patients (9%), but in none of the inflammatory skin lesions. The opposite picture, characterized by lack of antigen expression by the dermal T cells only, was not seen in any of the MF or inflammatory lesions. The absence of antigen expression by epidermal but not dermal T cells, which the authors have termed antigen discordance, was most prevalent for CD5, CD7, and TCR-beta, each being discordant in 6% to 7% of MF cases or patients tested. Among the MF biopsies showing antigen discordance, 14 of 15 biospies (93%) from 10 of 11 patients (91%) were discordant for two or more antigens. Antigen discordance was not an artifact of treatment, because none of the patients showing discordance was receiving treatment at the time of their initial discordant biopsy. The discordance was the only immunophenotypic abnormality detected in 8 of 15 (53%) of the discordant MF biopsies. Thus, this antigen discordance was an important diagnostic feature that allowed the immunophenotypic distinction of MF from a variety of inflammatory skin lesions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brousse N., Jarry A., Peuchmaur M., Gaulard P., D'Agay M. F., Guy-Grand D., Cerf-Bensussan N. Monoclonal antibody (HML-1) reactivity of T-cell lymphomas. Lancet. 1989 Nov 4;2(8671):1107–1108. doi: 10.1016/s0140-6736(89)91130-6. [DOI] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Lamberg S. I. Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep. 1979 Apr;63(4):725–728. [PubMed] [Google Scholar]
- Camerini D., James S. P., Stamenkovic I., Seed B. Leu-8/TQ1 is the human equivalent of the Mel-14 lymph node homing receptor. Nature. 1989 Nov 2;342(6245):78–82. doi: 10.1038/342078a0. [DOI] [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Triebel F., Hercend T. Characterization of human peripheral lymphocytes expressing the CD3-gamma/delta complex with anti-receptor monoclonal antibodies. J Immunol. 1988 Nov 15;141(10):3357–3360. [PubMed] [Google Scholar]
- Garcia C. F., Weiss L. M., Lowder J., Komoroske C., Link M. P., Levy R., Warnke R. A. Quantitation and estimation of lymphocyte subsets in tissue sections. Comparison with flow cytometry. Am J Clin Pathol. 1987 Apr;87(4):470–477. doi: 10.1093/ajcp/87.4.470. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of T lymphocytes in thymus and peripheral lymphoid tissues. Am J Pathol. 1985 Oct;121(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- Kansas G. S., Wood G. S., Fishwild D. M., Engleman E. G. Functional characterization of human T lymphocyte subsets distinguished by monoclonal anti-leu-8. J Immunol. 1985 May;134(5):2995–3002. [PubMed] [Google Scholar]
- Michie S. A., Abel E. A., Hoppe R. T., Warnke R. A., Wood G. S. Expression of T-cell receptor antigens in mycosis fungoides and inflammatory skin lesions. J Invest Dermatol. 1989 Jul;93(1):116–120. doi: 10.1111/1523-1747.ep12277376. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Griffiths C. E. Intraepidermal but not dermal T lymphocytes are positive for a cell-cycle-associated antigen (Ki-67) in mycosis fungoides. Am J Pathol. 1990 Feb;136(2):261–266. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Weiss L. M., Medeiros L. J., Wood G. S., Warnke R. A. Immunophenotypic criteria for the diagnosis of non-Hodgkin's lymphoma. Am J Pathol. 1987 Jul;128(1):181–201. [PMC free article] [PubMed] [Google Scholar]
- Ralfkiaer E., Wantzin G. L., Mason D. Y., Hou-Jensen K., Stein H., Thomsen K. Phenotypic characterization of lymphocyte subsets in mycosis fungoides. Comparison with large plaque parapsoriasis and benign chronic dermatoses. Am J Clin Pathol. 1985 Nov;84(5):610–619. doi: 10.1093/ajcp/84.5.610. [DOI] [PubMed] [Google Scholar]
- Schieferdecker H. L., Ullrich R., Weiss-Breckwoldt A. N., Schwarting R., Stein H., Riecken E. O., Zeitz M. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990 Apr 1;144(7):2541–2549. [PubMed] [Google Scholar]
- Sperling M., Kaudewitz P., Braun-Falco O., Stein H. Reactivity of T cells in mycosis fungoides exhibiting marked epidermotropism with the monoclonal antibody HML-1 that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1989 May;134(5):955–960. [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Penta A. C., Levine H. B., Freedman A. S. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990 Jan 15;144(2):532–540. [PubMed] [Google Scholar]
- Wood G. S., Abel E. A., Hoppe R. T., Warnke R. A. Leu-8 and Leu-9 antigen phenotypes: immunologic criteria for the distinction of mycosis fungoides from cutaneous inflammation. J Am Acad Dermatol. 1986 Jun;14(6):1006–1013. doi: 10.1016/s0190-9622(86)70124-2. [DOI] [PubMed] [Google Scholar]
- van der Putte S. C., Toonstra J., van Wichen D. F., van Unnik J. A., van Vloten W. A. Aberrant immunophenotypes in mycosis fungoides. Arch Dermatol. 1988 Mar;124(3):373–380. doi: 10.1001/archderm.124.3.373. [DOI] [PubMed] [Google Scholar]