Abstract
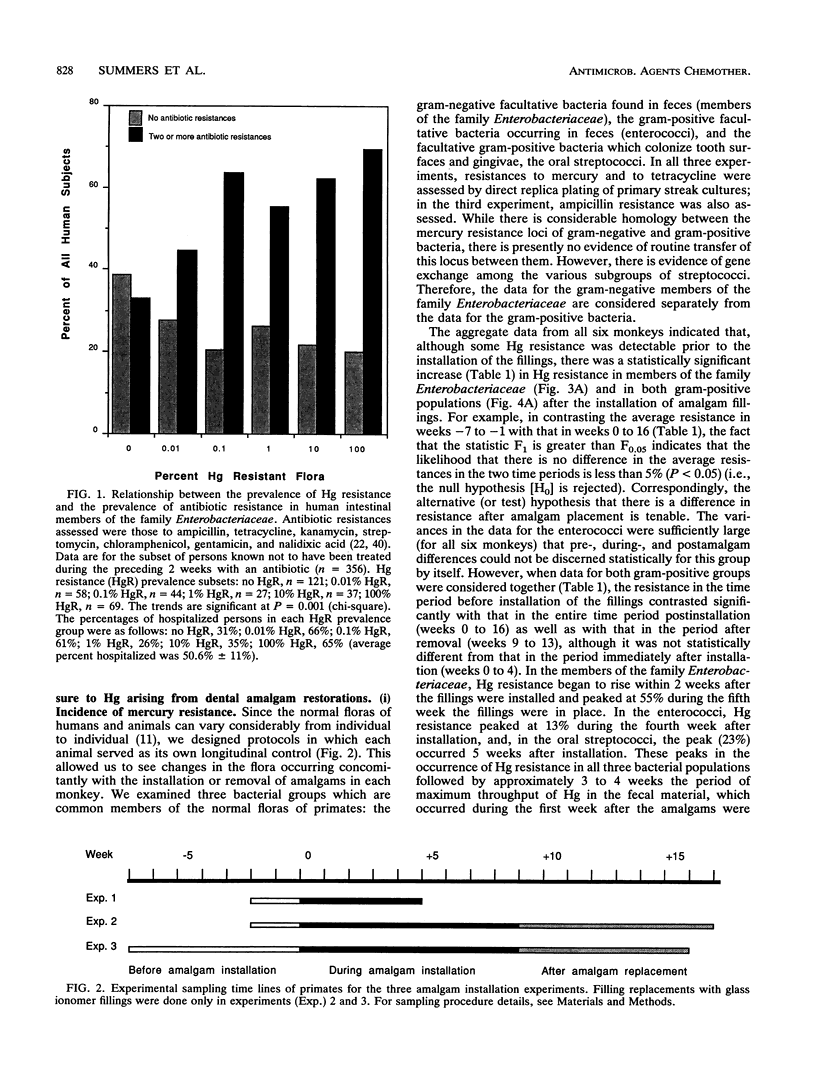
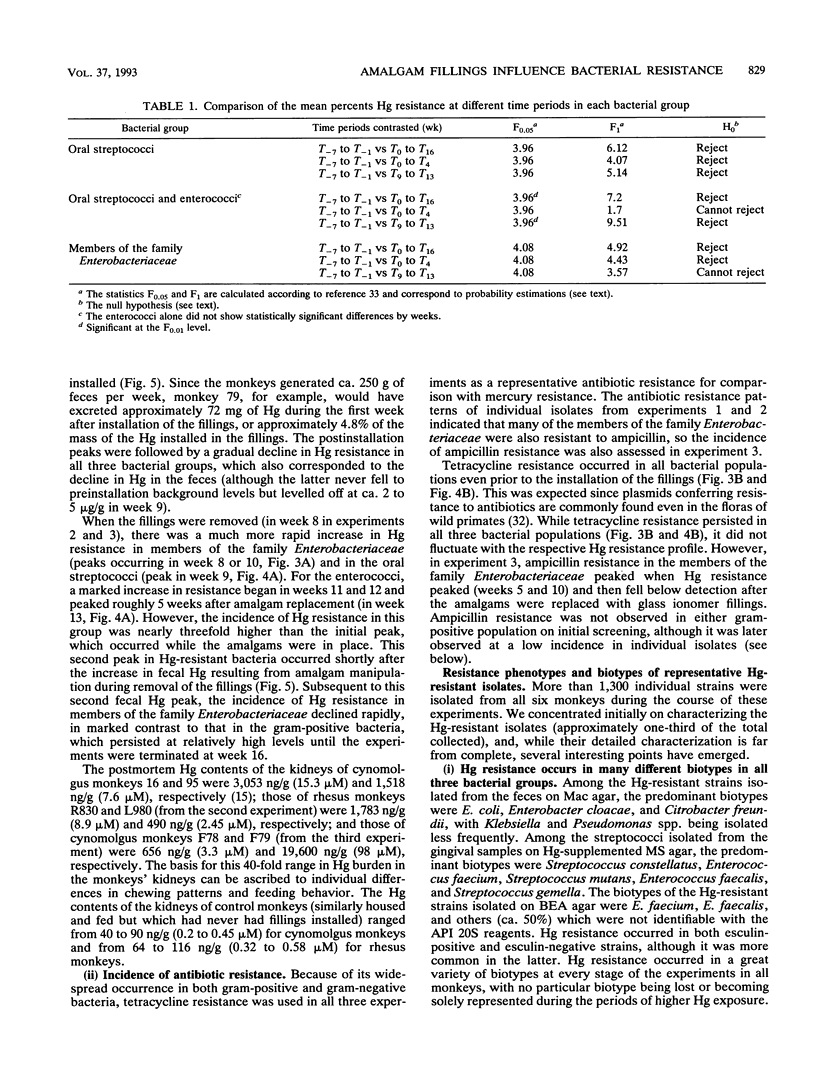
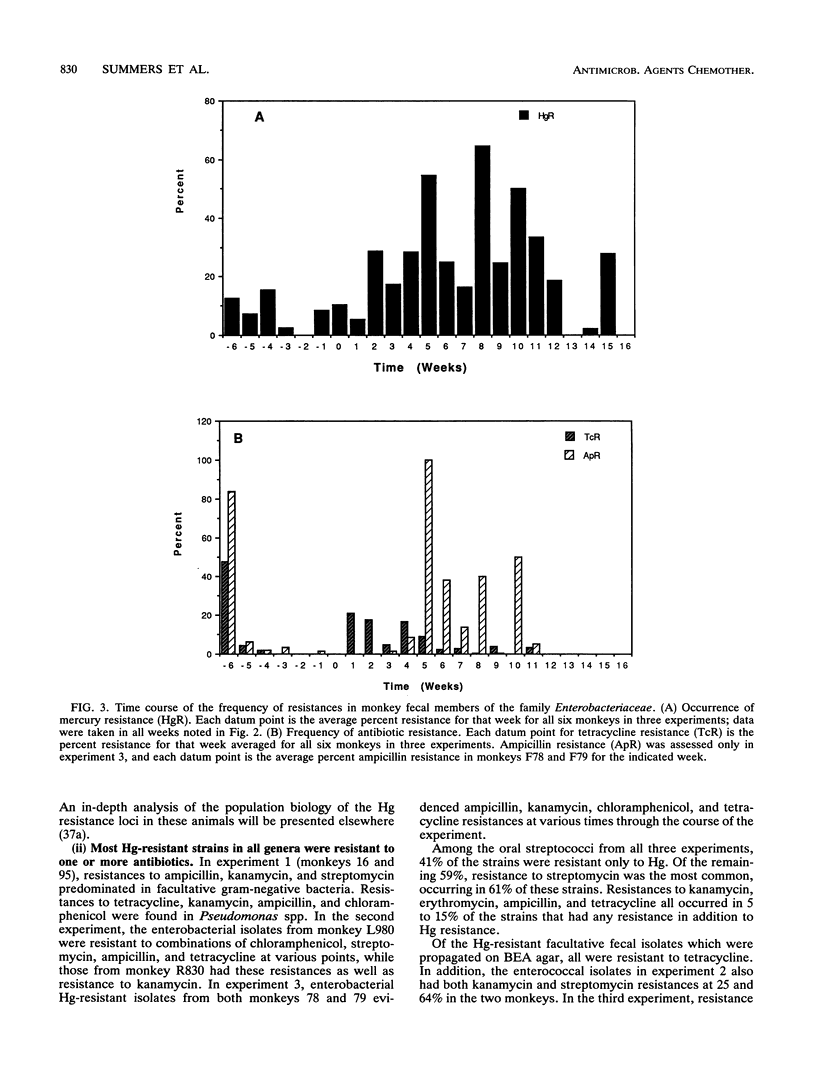
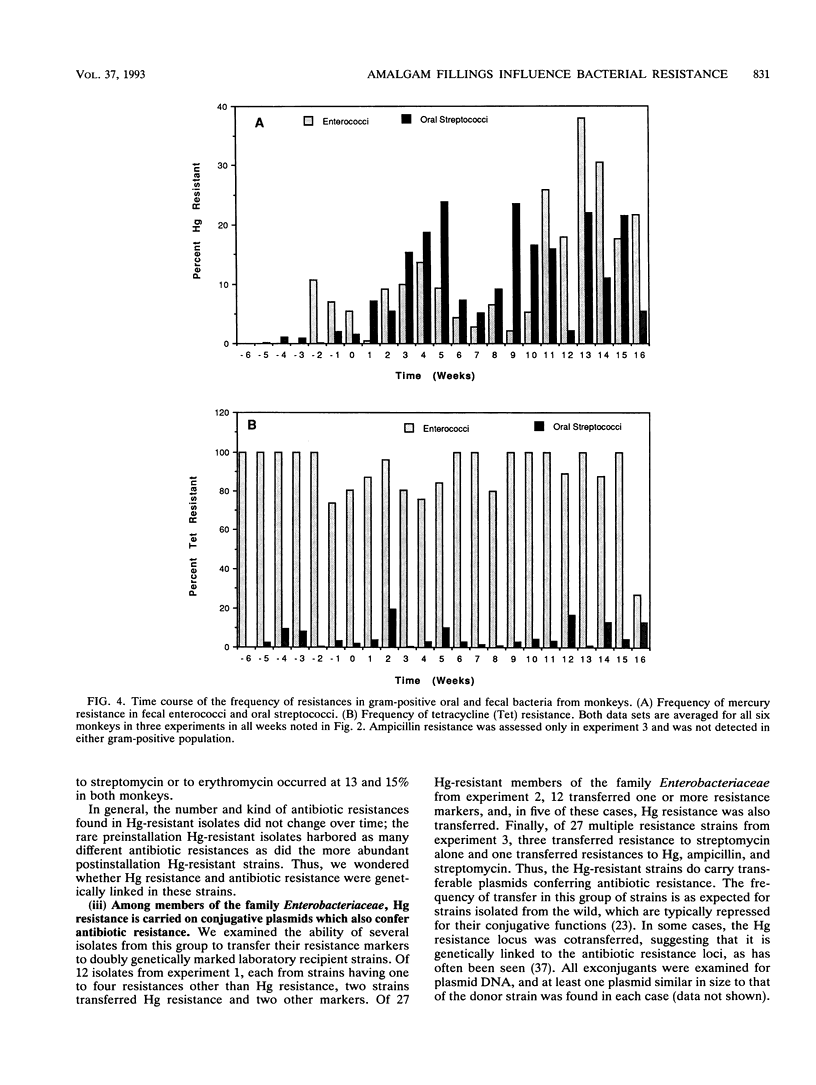
In a survey of 640 human subjects, a subgroup of 356 persons without recent exposure to antibiotics demonstrated that those with a high prevalence of Hg resistance in their intestinal floras were significantly more likely to also have resistance to two or more antibiotics. This observation led us to consider the possibility that mercury released from amalgam ("silver") dental restorations might be a selective agent for both mercury- and antibiotic-resistant bacteria in the oral and intestinal floras of primates. Resistances to mercury and to several antibiotics were examined in the oral and intestinal floras of six adult monkeys prior to the installation of amalgam fillings, during the time they were in place, and after replacement of the amalgam fillings with glass ionomer fillings (in four of the monkeys). The monkeys were fed an antibiotic-free diet, and fecal mercury concentrations were monitored. There was a statistically significant increase in the incidence of mercury-resistant bacteria during the 5 weeks following installation of the amalgam fillings and during the 5 weeks immediately following their replacement with glass ionomer fillings. These peaks in incidence of mercury-resistant bacteria correlated with peaks of Hg elimination (as high as 1 mM in the feces) immediately following amalgam placement and immediately after replacement of the amalgam fillings. Representative mercury-resistant isolates of three selected bacterial families (oral streptococci, members of the family Enterobacteriaceae, and enterococci) were also resistant to one or more antibiotics, including ampicillin, tetracycline, streptomycin, kanamycin, and chloramphenicol. While such mercury- and antibiotic-resistant isolates among the staphylococci, the enterococci, and members of the family Enterobacteriaceae have been described, this is the first report of mercury resistance in the oral streptococci. Many of the enterobacterial strains were able to transfer mercury and antibiotic resistances together to laboratory bacterial recipients, suggesting that the loci for these resistances are genetically linked. Our findings indicate that mercury released from amalgam fillings can cause an enrichment of mercury resistance plasmids in the normal bacterial floras of primates. Many of these plasmids also carry antibiotic resistance, implicating the exposure to mercury from dental amalgams in an increased incidence of multiple antibiotic resistance plasmids in the normal floras of nonmedicated subjects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkay T. Adaptation of aquatic microbial communities to hg stress. Appl Environ Microbiol. 1987 Dec;53(12):2725–2732. doi: 10.1128/aem.53.12.2725-2732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. G., First H. A. The toxicity of mercury in dental amalgam. CDA J. 1982 Jun;10(6):47–61. [PubMed] [Google Scholar]
- Begley T. P., Walts A. E., Walsh C. T. Bacterial organomercurial lyase: overproduction, isolation, and characterization. Biochemistry. 1986 Nov 4;25(22):7186–7192. doi: 10.1021/bi00370a063. [DOI] [PubMed] [Google Scholar]
- Begley T. P., Walts A. E., Walsh C. T. Mechanistic studies of a protonolytic organomercurial cleaving enzyme: bacterial organomercurial lyase. Biochemistry. 1986 Nov 4;25(22):7192–7200. doi: 10.1021/bi00370a064. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. D., Benediktsson H., Vimy M. J., Hooper D. E., Lorscheider F. L. Mercury from dental "silver" tooth fillings impairs sheep kidney function. Am J Physiol. 1991 Oct;261(4 Pt 2):R1010–R1014. doi: 10.1152/ajpregu.1991.261.4.R1010. [DOI] [PubMed] [Google Scholar]
- Cohen M. L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992 Aug 21;257(5073):1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- Corpet D. E. Antibiotic resistance from food. N Engl J Med. 1988 May 5;318(18):1206–1207. doi: 10.1056/nejm198805053181818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher G., Hørsted-Bindslev P., Rungby J. Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol. 1990 Jun;52(3):291–299. doi: 10.1016/0014-4800(90)90070-t. [DOI] [PubMed] [Google Scholar]
- Gilbert M. P., Summers A. O. The distribution and divergence of DNA sequences related to the Tn21 and Tn501 mer operons. Plasmid. 1988 Sep;20(2):127–136. doi: 10.1016/0147-619x(88)90015-7. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Galloway W. D., Clarkson T. W., Lorscheider F. L., Berlin M., Rowland A. S. Toxicity assessment of mercury vapor from dental amalgams. Fundam Appl Toxicol. 1992 Oct;19(3):319–329. doi: 10.1016/0272-0590(92)90169-i. [DOI] [PubMed] [Google Scholar]
- Hahn L. J., Kloiber R., Leininger R. W., Vimy M. J., Lorscheider F. L. Whole-body imaging of the distribution of mercury released from dental fillings into monkey tissues. FASEB J. 1990 Nov;4(14):3256–3260. doi: 10.1096/fasebj.4.14.2227216. [DOI] [PubMed] [Google Scholar]
- Hahn L. J., Kloiber R., Vimy M. J., Takahashi Y., Lorscheider F. L. Dental "silver" tooth fillings: a source of mercury exposure revealed by whole-body image scan and tissue analysis. FASEB J. 1989 Dec;3(14):2641–2646. doi: 10.1096/fasebj.3.14.2636872. [DOI] [PubMed] [Google Scholar]
- Hamlett N. V. Alteration of a salt marsh bacterial community by fertilization with sewage sludge. Appl Environ Microbiol. 1986 Oct;52(4):915–923. doi: 10.1128/aem.52.4.915-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khesin R. B., Karasyova E. V. Mercury-resistant plasmids in bacteria from a mercury and antimony deposit area. Mol Gen Genet. 1984;197(2):280–285. doi: 10.1007/BF00330974. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Marshall B., Schluederberg S., Rowse D., Davis J. High frequency of antimicrobial resistance in human fecal flora. Antimicrob Agents Chemother. 1988 Dec;32(12):1801–1806. doi: 10.1128/aac.32.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar M. R., Griffin N., Keyworth N. Pattern of antibiotic and heavy-metal ion resistance in recent hospital isolates of Staphylococcus aureus. Epidemiol Infect. 1987 Oct;99(2):343–347. doi: 10.1017/s0950268800067819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K. Bacterial resistances to inorganic mercury salts and organomercurials. Plasmid. 1992 Jan;27(1):4–16. doi: 10.1016/0147-619x(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Pla M. P., Mayer K. H., Kishi H., Gilleece E., Syvanen M., Hopkins J. D. Intercontinental spread of a new antibiotic resistance gene on an epidemic plasmid. Science. 1985 Oct 4;230(4721):87–88. doi: 10.1126/science.2994226. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Weissberg B. G., Dennison P. J. Mercury in human breath from dental amalgams. Bull Environ Contam Toxicol. 1985 Apr;34(4):459–468. doi: 10.1007/BF01609761. [DOI] [PubMed] [Google Scholar]
- Porter F. D., Silver S., Ong C., Nakahara H. Selection for mercurial resistance in hospital settings. Antimicrob Agents Chemother. 1982 Nov;22(5):852–858. doi: 10.1128/aac.22.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt J. W. Risk assessment of mercury exposure from dental amalgams. J Public Health Dent. 1988 Summer;48(3):172–177. doi: 10.1111/j.1752-7325.1988.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Rolland R. M., Hausfater G., Marshall B., Levy S. B. Antibiotic-resistant bacteria in wild primates: increased prevalence in baboons feeding on human refuse. Appl Environ Microbiol. 1985 Apr;49(4):791–794. doi: 10.1128/aem.49.4.791-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Summers A. O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992 May;174(10):3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svare C. W., Peterson L. C., Reinhardt J. W., Boyer D. B., Frank C. W., Gay D. D., Cox R. D. The effect of dental amalgams on mercury levels in expired air. J Dent Res. 1981 Sep;60(9):1668–1671. doi: 10.1177/00220345810600090601. [DOI] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimy M. J., Lorscheider F. L. Intra-oral air mercury released from dental amalgam. J Dent Res. 1985 Aug;64(8):1069–1071. doi: 10.1177/00220345850640080901. [DOI] [PubMed] [Google Scholar]
- Vimy M. J., Lorscheider F. L. Serial measurements of intra-oral air mercury: estimation of daily dose from dental amalgam. J Dent Res. 1985 Aug;64(8):1072–1075. doi: 10.1177/00220345850640081001. [DOI] [PubMed] [Google Scholar]



