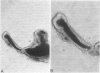
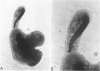
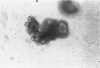
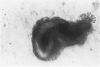
Abstract
Studies of the adenoma-carcinoma sequence in the colon and rectum have been limited by the paucity of experimental models of adenoma growth and progression. Progress recently was reported in the development of monolayer culture systems. The principal objective of this study was to develop a primary culture system for colorectal adenomas that would simulate three-dimensional in vivo growth. We used a calcium alginate encapsulation technique that was previously described for established tumor cell lines. Briefly, fresh resected specimens were washed, minced into small multicellular particles called microadenomas, and encapsulated in 1% calcium alginate pellets. The pellets were maintained in minimum essential medium containing 10% fetal bovine serum at 37 degrees C in humidified atmosphere of 95% air, 5% CO2. Ten of eleven adenomas, including six tubular, three tubulovillous, and one villous have been successfully cultured for 34 to 162 days. Cell viability was confirmed histologically by light and electron microscopy. The cells were characterized as epithelial by morphologic features and ultrastructural studies, which showed a high degree of cellular differentiation, including villous brush borders and many desmosomes. Both tubular and villuslike structures have been observed in vitro, correlating in some cases with the histology of the parent adenoma. Measurements of proliferative activity by [3H]thymidine autoradiography or immunohistochemical staining with the monoclonal antibody Ki-67 demonstrated growth fractions of 9% to 25%. A simple, highly efficient primary culture system was developed for the long-term maintenance of adenomas that promotes three-dimensional growth patterns and growth rates analogous to those seen in vivo. This model provides an opportunity to develop an experimental system for longitudinal studies of pathologic and molecular parameters in adenoma progression to carcinoma.
Full text
PDF
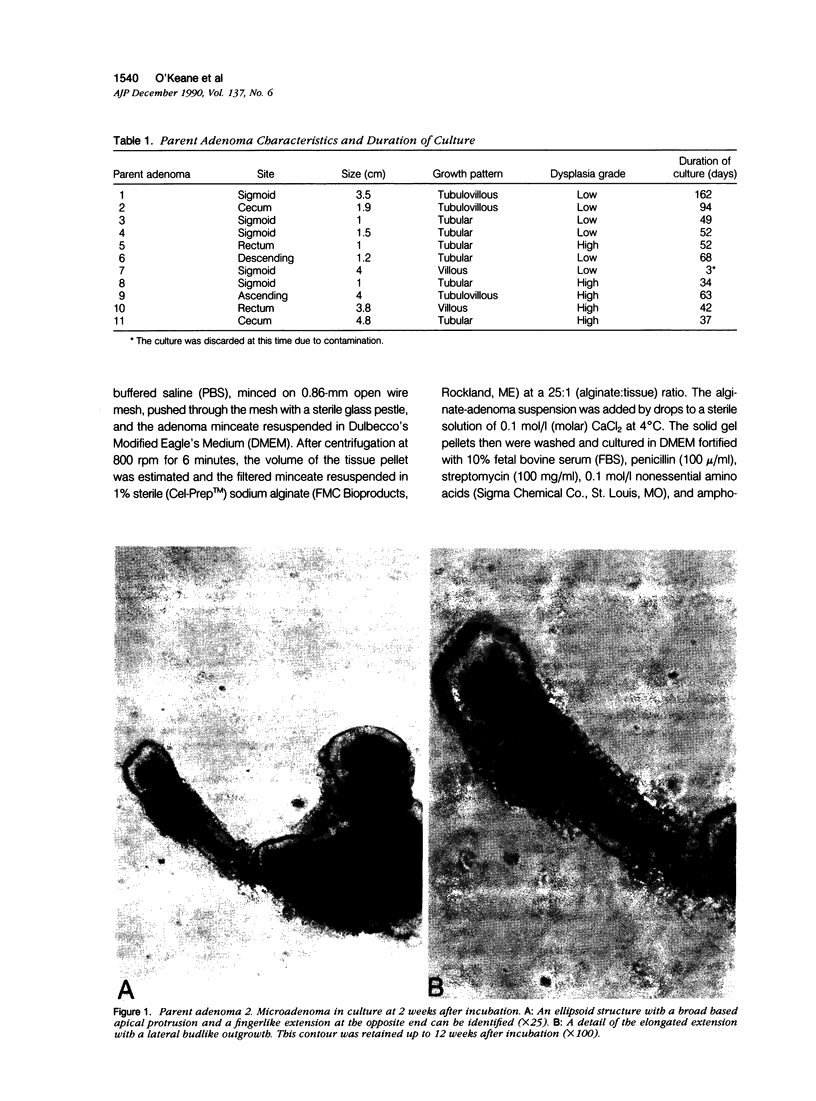
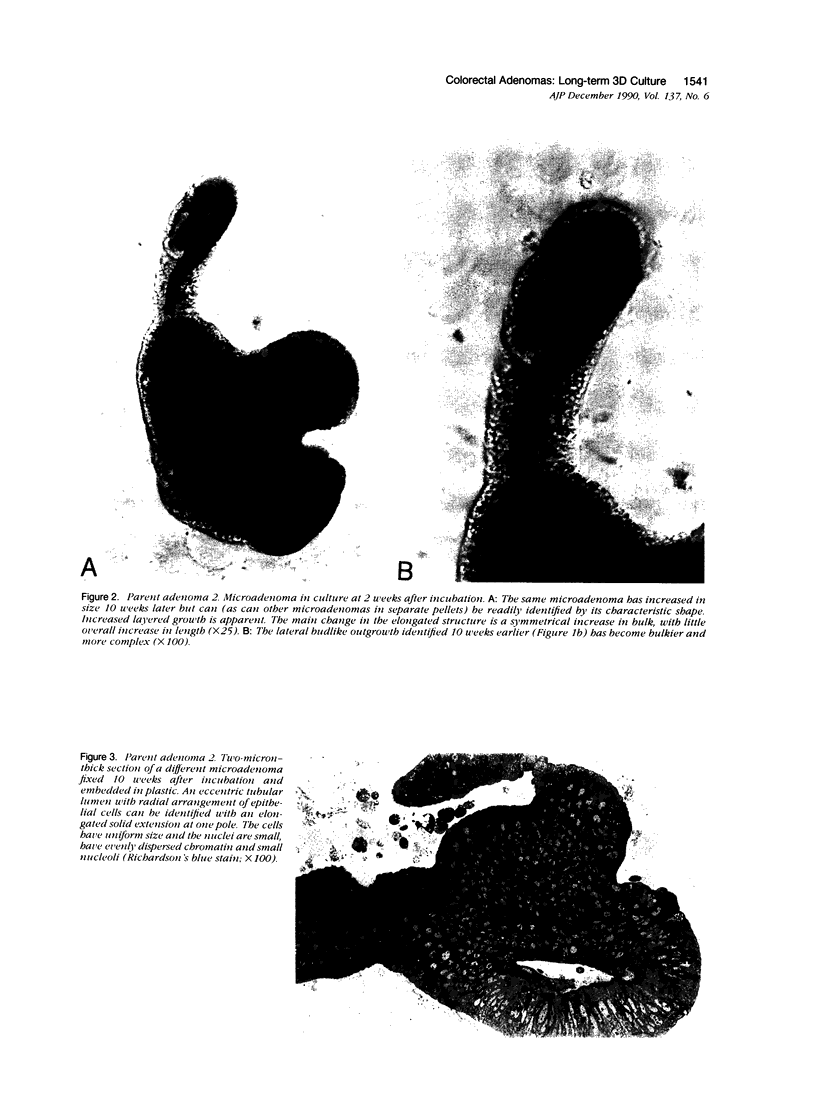




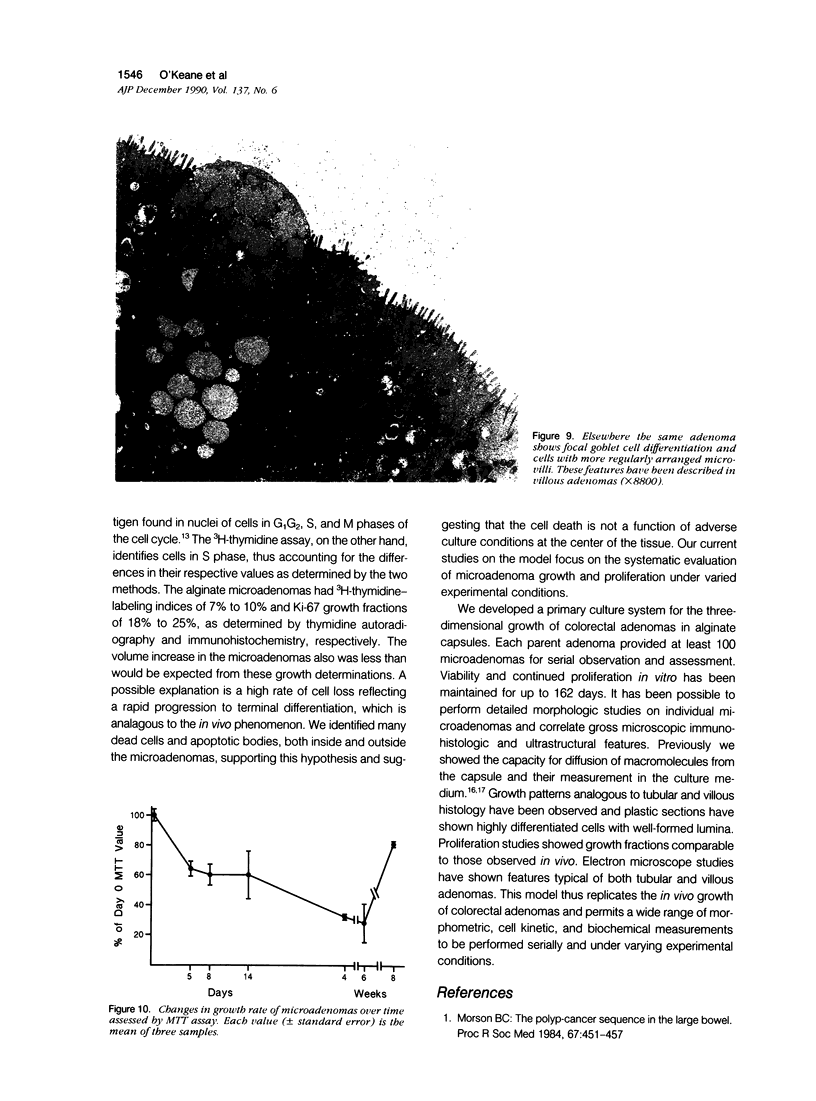

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMINSKI T. C., MCLEAN D. W. INCIDENCE AND DISTRIBUTION OF ADENOMATOUS POLYPS OF THE COLON AND RECTUM BASED ON 1,000 AUTOPSY EXAMINATIONS. Dis Colon Rectum. 1964 Jul-Aug;7:249–261. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- Camplejohn R. S., Bone G., Aherne W. Cell proliferation in rectal carcinoma and rectal mucosa. A stathmokinetic study. Eur J Cancer. 1973 Aug;9(8):577–581. doi: 10.1016/0014-2964(73)90148-5. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Clapp N. K., Lushbaugh C. C., Humason G. L., Gangaware B. L., Henke M. A. Natural history and pathology of colon cancer in Saguinus oedipus oedipus. Dig Dis Sci. 1985 Dec;30(12 Suppl):107S–113S. doi: 10.1007/BF01296988. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Maskens A. P. Significance of the labeling index and labeling distribution as kinetic parameters in colorectal mucosa of cancer patients and DMH treated animals. Cancer. 1982 Sep 15;50(6):1136–1141. doi: 10.1002/1097-0142(19820915)50:6<1136::aid-cncr2820500617>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Eide T. J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986 Aug 15;38(2):173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- Fenoglio C. M., Richart R. M., Kaye G. I. Comparative electron-microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. II. Variations in surface architecture found by scanning electron microscopy. Gastroenterology. 1975 Jul;69(1):100–109. [PubMed] [Google Scholar]
- Friedman E. A., Higgins P. J., Lipkin M., Shinya H., Gelb A. M. Tissue culture of human epithelial cells from benign colonic tumors. In Vitro. 1981 Jul;17(7):632–644. doi: 10.1007/BF02618462. [DOI] [PubMed] [Google Scholar]
- Friedman E. A., Steinberg M. Disrupted communication between late-stage premalignant human colon epithelial cells by 12-O tetradecanoylphorbol-13-acetate. Cancer Res. 1982 Dec;42(12):5096–5105. [PubMed] [Google Scholar]
- Friedman E., Isaksson P., Rafter J., Marian B., Winawer S., Newmark H. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res. 1989 Feb 1;49(3):544–548. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Hirtenstein M., Clark J., Lindgren G., Vretblad P. Microcarriers for animal cell culture: a brief review of theory and practice. Dev Biol Stand. 1980;46:109–116. [PubMed] [Google Scholar]
- IMAI H., SAITO S., STEIN A. A. ULTRASTRUCTURE OF ADENOMATOUS POLYPS AND VILLOUS ADENOMAS OF THE LARGE INTESTINE. Gastroenterology. 1965 Feb;48:188–197. [PubMed] [Google Scholar]
- Johnston P. G., O'Brien M. J., Dervan P. A., Carney D. N. Immunohistochemical analysis of cell kinetic parameters in colonic adenocarcinomas, adenomas, and normal mucosa. Hum Pathol. 1989 Jul;20(7):696–700. doi: 10.1016/0046-8177(89)90158-5. [DOI] [PubMed] [Google Scholar]
- Kaye G. I., Fenoglio C. M., Pascal R. R., Lane N. Comparative electron microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. Variations in cellular structure relative to the process of epithelial differentiation. Gastroenterology. 1973 May;64(5):926–945. [PubMed] [Google Scholar]
- Kupchik H. Z., Langer R. S., Haberern C., El Deriny S., O'Brien M. A new method for the three-dimensional in vitro growth of human cancer cells. Exp Cell Res. 1983 Sep;147(2):454–460. doi: 10.1016/0014-4827(83)90228-8. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Harte P., Deasy J., Ross D., Lahey S., Steele G., Jr Evidence for an adenoma-carcinoma sequence in dimethylhydrazine-induced neoplasms of rat intestinal epithelium. Am J Pathol. 1983 Feb;110(2):230–235. [PMC free article] [PubMed] [Google Scholar]
- Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974 Jun;67(6 Pt 1):451–457. [PMC free article] [PubMed] [Google Scholar]
- Niles R. M., Wilhelm S. A., Steele G. D., Jr, Burke B., Christensen T., Dexter D., O'Brien M. J., Thomas P., Zamcheck N. Isolation and characterization of an undifferentiated human colon carcinoma cell line (MIP-101). Cancer Invest. 1987;5(6):545–552. doi: 10.3109/07357908709020314. [DOI] [PubMed] [Google Scholar]
- O'Brien M. J. Cancer of the colon and rectum: current concepts of aetiology and pathogenesis. Ir J Med Sci. 1988 Jul;157(7 Suppl):5–15. [PubMed] [Google Scholar]
- O'Brien M. J., Winawer S. J., Zauber A. G., Gottlieb L. S., Sternberg S. S., Diaz B., Dickersin G. R., Ewing S., Geller S., Kasimian D. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990 Feb;98(2):371–379. [PubMed] [Google Scholar]
- Paraskeva C., Buckle B. G., Sheer D., Wigley C. B. The isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984 Jul 15;34(1):49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- Willson J. K., Bittner G. N. In vitro models of human colonic adenomatous polyps. Prog Clin Biol Res. 1988;279:347–356. [PubMed] [Google Scholar]
- Willson J. K., Bittner G. N., Oberley T. D., Meisner L. F., Weese J. L. Cell culture of human colon adenomas and carcinomas. Cancer Res. 1987 May 15;47(10):2704–2713. [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deriny S. E., O'Brien M. J., Christensen T. G., Kupchik H. Z. Ultrastructural differentiation and CEA expression of butyrate-treated human pancreatic carcinoma cells. Pancreas. 1987;2(1):25–33. doi: 10.1097/00006676-198701000-00004. [DOI] [PubMed] [Google Scholar]