Abstract
The local injection of pure inflammatory mediators induces venular leakage. To test the effect of endogenous mediators from dying tissue on vascular leakage, the authors devised an experimental model simulating an infarct, whereby living vessels would be exposed to fragments of organs undergoing aseptic necrosis. Tissues from donor rats were implanted aseptically in the cremasteric sac. Control rats were implanted with materials deemed to be as close as possible to nonirritating: boiled tissues and spheres of Teflon or glass. At different points the rats were injected intravenously with carbon black and killed an hour later. Whole cremaster mounts showed that vascular labeling was strictly venular up to 8 hours, mixed with capillary labeling between 12 and 24 hours, and mainly or exclusively capillary at 48 hours. Histology showed an acute inflammatory infiltrate in the labeled areas. A similar but weaker labeling pattern accompanied by milder inflammation was seen in controls. These results indicate that the vascular leakage in aseptic inflammation is biphasic, first venular, then capillary; and that the capillary phase is induced by the inflammatory reaction itself, possibly through a form of diffuse angiogenesis.
Full text
PDF

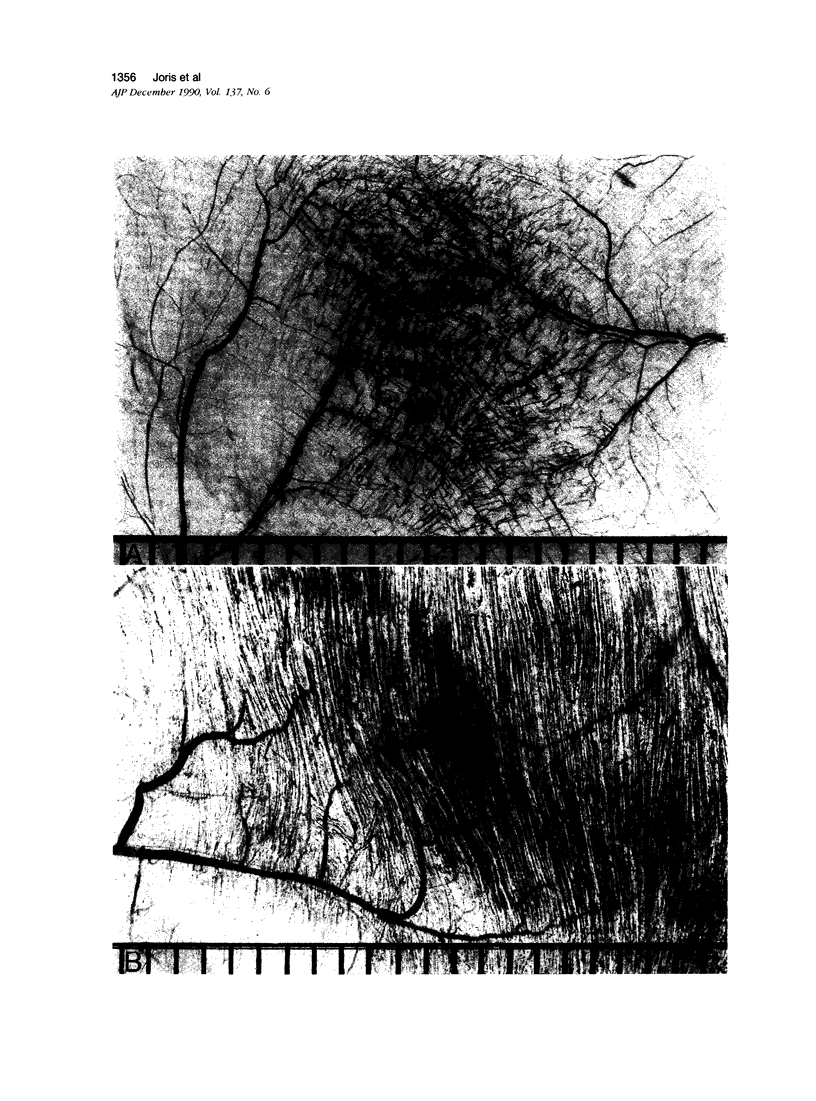


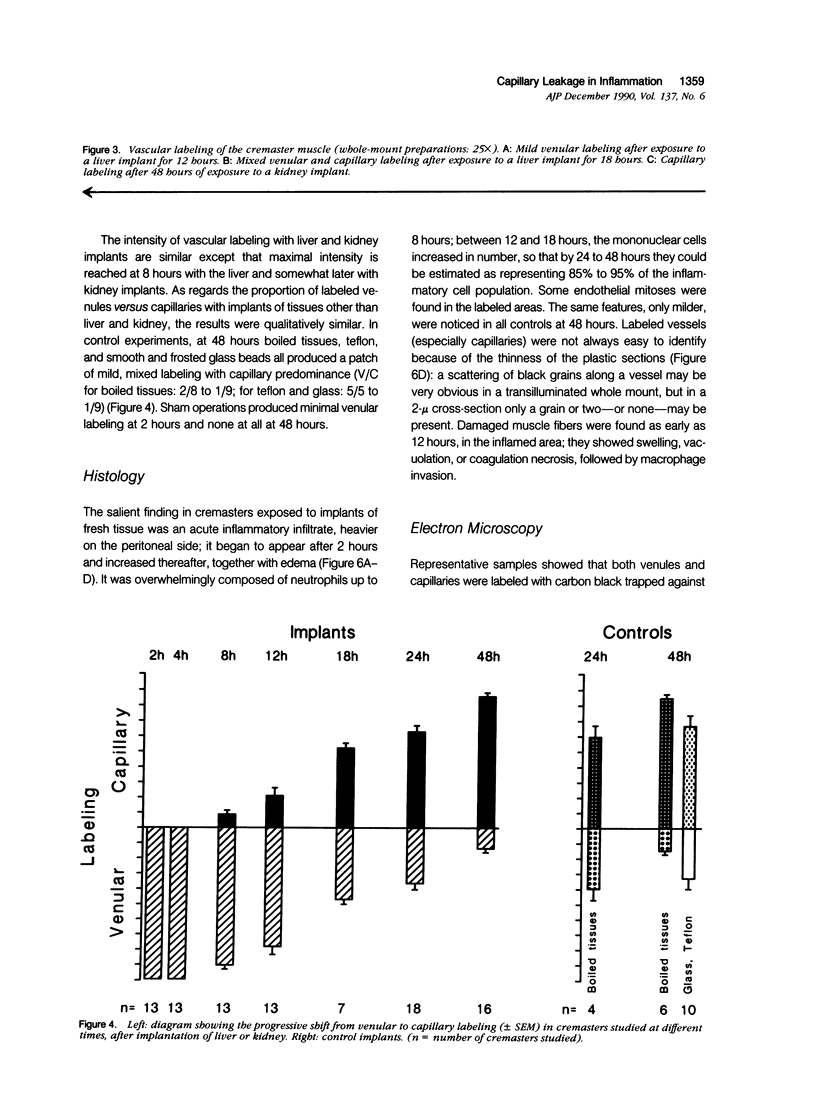



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTRAN R. S., MAJNO G. A LIGHT AND ELECTRON MICROSCOPIC ANALYSIS OF VASCULAR INJURY. Ann N Y Acad Sci. 1964 Aug 27;116:750–764. doi: 10.1111/j.1749-6632.1964.tb52543.x. [DOI] [PubMed] [Google Scholar]
- COTRAN R. S., MAJNO G. THE DELAYED AND PROLONGED VASCULAR LEAKAGE IN INFLAMMATION. I. TOPOGRAPHY OF THE LEAKING VESSELS AFTER THERMAL INJURY. Am J Pathol. 1964 Aug;45:261–281. [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S. Delayed and prolonged vascular leakage in inflammation. 3. Immediate and delayed vascular reactions in skeletal muscle. Exp Mol Pathol. 1967 Apr;6(2):143–155. doi: 10.1016/0014-4800(67)90052-4. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. Studies on inflammation. Ultrastructure of the prolonged vascular response induced by Clostridium oedematiens toxin. Lab Invest. 1967 Jul;17(1):39–60. [PubMed] [Google Scholar]
- Heltianu C., Simionescu M., Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982 May;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Hanahan D. J., Pinckard R. N. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984 Jan;50(1):16–25. [PubMed] [Google Scholar]
- Hurley J. V., Ham K. N., Ryan G. B. The mechanism of the delayed prolonged phase of increased vascular permeability in mild thermal injury in the rat. J Pathol Bacteriol. 1967 Jul;94(1):1–12. doi: 10.1002/path.1700940102. [DOI] [PubMed] [Google Scholar]
- Hurley J. V., Jago M. V. Delayed and prolonged vascular leakage in inflammation: the effects of dehydromonocrotaline on blood vessels in the rat cremaster. Pathology. 1976 Jan;8(1):7–20. doi: 10.3109/00313027609094419. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G., Corey E. J., Lewis R. A. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol. 1987 Jan;126(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- Krizek T. J., Robson M. C. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975 Nov;130(5):579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- Lin S. J., Jan K. M., Weinbaum S., Chien S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis. 1989 Mar-Apr;9(2):230–236. doi: 10.1161/01.atv.9.2.230. [DOI] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus L. M., Kolb W. P., Crawford M. H., O'Rourke R. A., Grover F. L., Pinckard R. N. Complement localization in ischemic baboon myocardium. Lab Invest. 1983 Apr;48(4):436–447. [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982 Apr 15;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- WELLS F. R., MILES A. A. SITE OF THE VASCULAR RESPONSE TO THERMAL INJURY. Nature. 1963 Dec 7;200:1015–1016. doi: 10.1038/2001015a0. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Kolb W. P. Complement-independent activation of the fifth component (C5) of human complement: limited trypsin digestion resulting in the expression of biological activity. J Immunol. 1982 May;128(5):2209–2216. [PubMed] [Google Scholar]
- Wiener J., Lattes R. G., Spiro D. An electron microscopic study of leukocyte emigration and vascular permeability in tuberculin sensitivity. Am J Pathol. 1967 Mar;50(3):485–521. [PMC free article] [PubMed] [Google Scholar]
- Willms-Kretschmer K., Flax M. H., Cotran R. S. The fine structure of the vascular response in hapten-specific delayed hypersensitivity and contact dermatitis. Lab Invest. 1967 Sep;17(3):334–349. [PubMed] [Google Scholar]
- Willms-Kretschmer K., Majno G. Ischemia of the skin. Electron microscopic study of vascular injury. Am J Pathol. 1969 Mar;54(3):327–353. [PMC free article] [PubMed] [Google Scholar]
- de Almeida O. P., Böhm G. M. Vascular permeability in the rat gingiva. A model of vessel response in chronic inflammation. J Pathol. 1979 Jan;127(1):27–34. doi: 10.1002/path.1711270105. [DOI] [PubMed] [Google Scholar]