Abstract
Structural characterization of the bacterial channel, AmtB, provides a glimpse of how members of its family might control the protonated state of permeant ammonium to allow for its selective passage across the membrane. In a recent study, we employed a combination of simulation techniques that suggested ammonium is deprotonated and reprotonated near dehydrative phenylalanine landmarks (F107 and F31, respectively) during its passage from the periplasm to the cytoplasm. At these landmarks, ammonium is forced to maintain a critical number (∼3) of hydrogen bonds, suggesting that the channel controls ammonium (de)protonation by controlling its coordination/hydration. In the work presented here, a free energy-based analysis of ammonium hydration in dilute aqueous solution indicates, explicitly, that at biological pH, the transition from ammonium (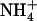 ) to ammonia (NH3) occurs when these species are constrained to donate three hydrogen bonds or less. This result demonstrates the viability of the proposal that AmtB indirectly controls ammonium (de)protonation by directly controlling its hydration.
) to ammonia (NH3) occurs when these species are constrained to donate three hydrogen bonds or less. This result demonstrates the viability of the proposal that AmtB indirectly controls ammonium (de)protonation by directly controlling its hydration.
AmtB exists in the membrane as a homotrimer. Each monomer of this protein forms a channel that passively transports ammonium (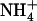 ) in the form of its “gas” ammonia (NH3) intermediate across the membranes of bacteria; for conciseness we will henceforth refer to both
) in the form of its “gas” ammonia (NH3) intermediate across the membranes of bacteria; for conciseness we will henceforth refer to both 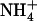 and NH3 species, together, as Am. Structural models of AmtB resulting from x-ray diffraction (1,2) have provided initial configurations for a plethora of computational (3–10,13) studies aimed at understanding this channel's mechanistic aspects and implications for homologous human counterparts.
and NH3 species, together, as Am. Structural models of AmtB resulting from x-ray diffraction (1,2) have provided initial configurations for a plethora of computational (3–10,13) studies aimed at understanding this channel's mechanistic aspects and implications for homologous human counterparts.
The center of an AmtB monomer forms a narrow hydrophobic pore (lumen) connecting cytoplasmic and periplasmic vestibules, both accessible to aqueous solution. Diffraction studies revealed an 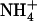 binding site in the cytoplasmic vestibule (site Am1 (1,2)) where the cation donates hydrogen bonds to the backbone carbonyl group of A162, the side-chain hydroxyl oxygen of S219, and ∼2–3 water molecules (3,5,7). Aromatic groups (F107 and F215) form a floor for site Am1, capping the hydrophobic lumen to help prevent entrance of water from the periplasm (see Fig. 1). These aromatic groups rotate at low free energy cost to allow translocation of Am (3,5,7) under the influence of an electrochemical gradient.
binding site in the cytoplasmic vestibule (site Am1 (1,2)) where the cation donates hydrogen bonds to the backbone carbonyl group of A162, the side-chain hydroxyl oxygen of S219, and ∼2–3 water molecules (3,5,7). Aromatic groups (F107 and F215) form a floor for site Am1, capping the hydrophobic lumen to help prevent entrance of water from the periplasm (see Fig. 1). These aromatic groups rotate at low free energy cost to allow translocation of Am (3,5,7) under the influence of an electrochemical gradient.
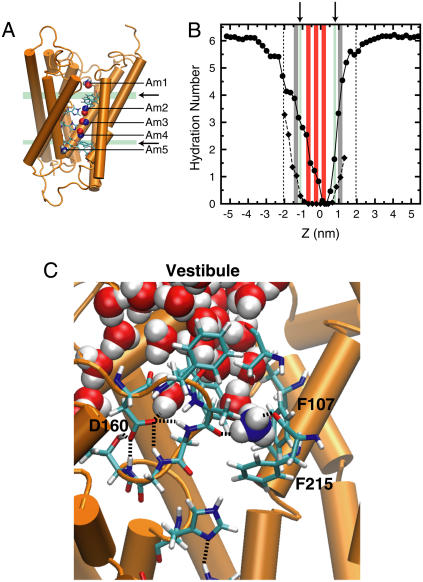
FIGURE 1.
(A) Synopsis of binding sites and deprotonation regions. Some helices have been removed from the protein (orange) to allow visibility of the binding sites. The positions of the x-ray sites are shown as red spheres, and Am binding sites derived from simulation (3) are shown as either blue spheres (for NH3) or blue spheres with white hydrogen atoms (for 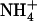 ). (De)protonation regions are marked in green (see arrows). The periplasmic and cytoplasmic (de)protonation regions coincide with the phenyl groups of F107 and F31, respectively. (B) Hydration of NH3 (diamonds with dashed line) and
). (De)protonation regions are marked in green (see arrows). The periplasmic and cytoplasmic (de)protonation regions coincide with the phenyl groups of F107 and F31, respectively. (B) Hydration of NH3 (diamonds with dashed line) and 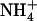 (circles with solid line) as a function of the transport axis (Z). The origin coincides with the center of mass of the AmtB trimer (Z > 0 is the periplasmic membrane leaflet, and Z < 0 is the cytoplasmic). Dotted lines denote lipid phosphate positions, gray bars mark sites Am1 and Am5, red bars mark sites Am2-4, and green bars (see arrows) mark (de)protonation regions (Am equivalence points). (C) Proposed equivalence point for
(circles with solid line) as a function of the transport axis (Z). The origin coincides with the center of mass of the AmtB trimer (Z > 0 is the periplasmic membrane leaflet, and Z < 0 is the cytoplasmic). Dotted lines denote lipid phosphate positions, gray bars mark sites Am1 and Am5, red bars mark sites Am2-4, and green bars (see arrows) mark (de)protonation regions (Am equivalence points). (C) Proposed equivalence point for 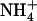 deprotonation near the periplasmic end of the lumen. Here,
deprotonation near the periplasmic end of the lumen. Here, 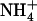 is stripped to three hydrogen bonds (donated to carbonyl groups of A162 and F215 and one water), and has full access to vestibular water, continuously connected to bulk solution, to allow the escape of a proton to the bulk in the form of hydronium ion.
is stripped to three hydrogen bonds (donated to carbonyl groups of A162 and F215 and one water), and has full access to vestibular water, continuously connected to bulk solution, to allow the escape of a proton to the bulk in the form of hydronium ion.
In the presence of AmSO4, the x-ray structure (1) displayed three luminal binding sites (Am2, Am3, and Am4—see Fig. 1 A), where Am interacts closely with His residues (H168 and H318). Calculations of the apparent pKa of luminal Am (3,10) indicate that these sites may only be occupied by neutral NH3. As such, it would appear that the disallowance of permanently charged species in the lumen is the most Am-selective feature of AmtB. An aromatic group (F31) just below site Am4 helps to prevent hydration of the lumen, and provides a low free energy barrier for NH3 passage to the cytoplasmic vestibule (Fig. 1, A and B). Just below the lumen, a fifth site (Am5) was revealed by a molecular dynamics (MD) study (3). At this site, calculations of the apparent pKa (3) suggest Am must exist in its protonated form, where it donates hydrogen bonds to a carboxyl oxygen of D313, the hydroxyl oxygen of S263, and surrounding water (Fig. 1, A and B).
Combining knowledge of experimental and computational results (1–3,10), it appears that AmtB deprotonates 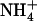 between sites Am1–2, and reprotonates NH3 between sites Am4–5 to allow Am flux toward the cytoplasm. However, it is difficult to determine, experimentally, how the channel controls these (de)protonation events. Computational studies, though they should help clarify the (de)protonation mechanism, have proposed disparate explanations (3–5,7). Lin et al. (5) and Nygaard et al. (7) both proposed that a highly conserved Asp residue (D160), whose mutation is known to destroy AmtB's transport capability (11), plays a key role in
between sites Am1–2, and reprotonates NH3 between sites Am4–5 to allow Am flux toward the cytoplasm. However, it is difficult to determine, experimentally, how the channel controls these (de)protonation events. Computational studies, though they should help clarify the (de)protonation mechanism, have proposed disparate explanations (3–5,7). Lin et al. (5) and Nygaard et al. (7) both proposed that a highly conserved Asp residue (D160), whose mutation is known to destroy AmtB's transport capability (11), plays a key role in 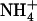 deprotonation. Lin et al. (5) observed that water forms a hydrogen bonded network between
deprotonation. Lin et al. (5) observed that water forms a hydrogen bonded network between 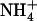 at Am1 and the carboxylate of D160. This led them to suggest that the charged carboxylate drives deprotonation at site Am1, and accepts a proton donated by
at Am1 and the carboxylate of D160. This led them to suggest that the charged carboxylate drives deprotonation at site Am1, and accepts a proton donated by 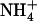 using hydronium as an intermediate. On the other hand, Nygaard et al. (7) proposed that deprotonation occurs near site Am2, after
using hydronium as an intermediate. On the other hand, Nygaard et al. (7) proposed that deprotonation occurs near site Am2, after 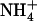 moves from Am1 across the stacked (F107/F215) aromatic moieties. In this configuration, it was suggested that
moves from Am1 across the stacked (F107/F215) aromatic moieties. In this configuration, it was suggested that 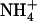 donates a proton to D160 via the backbone carbonyl group of A162 and the amide N–H of G163 using an imidic acid mechanism.
donates a proton to D160 via the backbone carbonyl group of A162 and the amide N–H of G163 using an imidic acid mechanism.
Luzhkov et al. (10) presented results that would suggest that D160 does not function as a proton acceptor. Rather, their calculations showed that the apparent pKa of D160's carboxylate is downshifted (from its standard value of ∼3.9) by 0.3–5.1 units when site Am1 is unoccupied. When 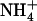 occupies Am1, the apparent pKa of D160 shifts even further downward by 9.2 units, making its protonation effectively impossible. Our own results (3), as well as those of Luzhkov et al., showed that D160 is engaged in persistent hydrogen bonds with the protein, and that the negative charge of D160 stabilizes Am in its protonated form, shifting its apparent pKa upward by ∼4 units. Taken together, these results indicate that the importance of D160, as evidenced by mutational studies (11), is more likely due to recruitment of
occupies Am1, the apparent pKa of D160 shifts even further downward by 9.2 units, making its protonation effectively impossible. Our own results (3), as well as those of Luzhkov et al., showed that D160 is engaged in persistent hydrogen bonds with the protein, and that the negative charge of D160 stabilizes Am in its protonated form, shifting its apparent pKa upward by ∼4 units. Taken together, these results indicate that the importance of D160, as evidenced by mutational studies (11), is more likely due to recruitment of 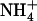 from the periplasm and stabilizing its binding at site Am1 rather than accepting a proton as suggested by Lin et al. and Nygaard et al.
from the periplasm and stabilizing its binding at site Am1 rather than accepting a proton as suggested by Lin et al. and Nygaard et al.
Recently we utilized a combination of MD simulation techniques (3), showing that the equivalence points for Am (de)protonation coincide with the periplasmic and cytoplasmic phenyl groups of F107 and F31, respectively (Fig. 1 A). Near these specific regions, Am was seen to be stripped to ∼3 or fewer hydrogen bonds (Fig. 1 B). At the periplasmic (de)protonation site (Fig. 1, B and C), near F107, 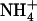 may donate two hydrogen bonds to protein and ∼1 to water. At the cytoplasmic (de)protonation site, it appears that water provides all ∼3 hydrogen bonds (Fig. 1 B). Given that, a), water ionizes more easily than a carbonyl group, b), the carboxylate of D160 is persistently engaged in hydrogen bonds with the protein that shift its apparent pKa downward (10), and c), at both equivalence points, Am has full access to vestibular water, we proposed that water is the only plausible proton acceptor for
may donate two hydrogen bonds to protein and ∼1 to water. At the cytoplasmic (de)protonation site, it appears that water provides all ∼3 hydrogen bonds (Fig. 1 B). Given that, a), water ionizes more easily than a carbonyl group, b), the carboxylate of D160 is persistently engaged in hydrogen bonds with the protein that shift its apparent pKa downward (10), and c), at both equivalence points, Am has full access to vestibular water, we proposed that water is the only plausible proton acceptor for  After accepting this proton, it is most likely that the proton escapes to the periplasm in the form of hydronium.
After accepting this proton, it is most likely that the proton escapes to the periplasm in the form of hydronium.
Our previous study showed a clear correlation between the protonated form of Am and the number of available hydrogen bonds. However, we did not directly demonstrate that the channel need only constrain Am to ∼3 or fewer hydrogen bonds to deprotonate  Such a demonstration would require a computational experiment that rules out other effects, such as the local electric field imposed by the channel at the (de)protonation regions. To address this issue in a most general way, we performed simulations of both
Such a demonstration would require a computational experiment that rules out other effects, such as the local electric field imposed by the channel at the (de)protonation regions. To address this issue in a most general way, we performed simulations of both 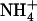 and NH3 in dilute aqueous solution (see Supplementary Material). These simple simulations allowed us to isolate and directly probe the dependence of Am's apparent pKa on coordination (hydrogen-bond) number.
and NH3 in dilute aqueous solution (see Supplementary Material). These simple simulations allowed us to isolate and directly probe the dependence of Am's apparent pKa on coordination (hydrogen-bond) number.
In the spirit of previous work (12), we performed a free energy characterization of solute (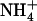 and NH3) hydration preferences based upon population analysis from MD trajectories. From this analysis, we derived the apparent pKa of Am as a function of its coordination number as follows (see Supplementary Material):
and NH3) hydration preferences based upon population analysis from MD trajectories. From this analysis, we derived the apparent pKa of Am as a function of its coordination number as follows (see Supplementary Material):
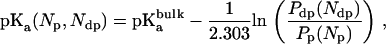 |
where, Px(Nx) is the probability that the coordination (hydrogen bond) number around Am in the state x is Nx (x = dp for “deprotonated” Am, or NH3, and x = p for “protonated” Am, or 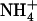 ), and
), and 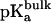 is the pKa of
is the pKa of 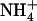 in bulk aqueous solution.
in bulk aqueous solution.
The resulting apparent pKa profile is shown in Fig. 2. This analysis indicates that if a local environment provides only ∼3 or fewer hydrogen bonds, Am will be favored in its deprotonated form, NH3. The equivalence point between 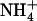 and NH3, itself, appears to occur near the midpoint between three and four available acceptors. In tandem with previous results (3,10), this indicates that the functional role of D160 is to allow for AmtB's structural and electrostatic ability to recruit
and NH3, itself, appears to occur near the midpoint between three and four available acceptors. In tandem with previous results (3,10), this indicates that the functional role of D160 is to allow for AmtB's structural and electrostatic ability to recruit 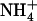 from the periplasm, and not to drive the deprotonation of
from the periplasm, and not to drive the deprotonation of  Since Fig. 2 describes the local pKa of Am in an isotropic medium—that of pure water—the analysis shows that loss of a proton from
Since Fig. 2 describes the local pKa of Am in an isotropic medium—that of pure water—the analysis shows that loss of a proton from 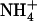 occurs with ∼3 hydrogen bonds regardless of any external field provided by AmtB at the calculated equivalence points shown in Fig. 1. The result we show here appears to be independent of the force field chosen to describe Am or water (Supplementary Material, Fig. S1), and suggests that AmtB's control over Am hydration, or equivalently, the number of hydrogen bonds, is the sole control over (de)protonation provided by the protein, as we have suggested (3).
occurs with ∼3 hydrogen bonds regardless of any external field provided by AmtB at the calculated equivalence points shown in Fig. 1. The result we show here appears to be independent of the force field chosen to describe Am or water (Supplementary Material, Fig. S1), and suggests that AmtB's control over Am hydration, or equivalently, the number of hydrogen bonds, is the sole control over (de)protonation provided by the protein, as we have suggested (3).
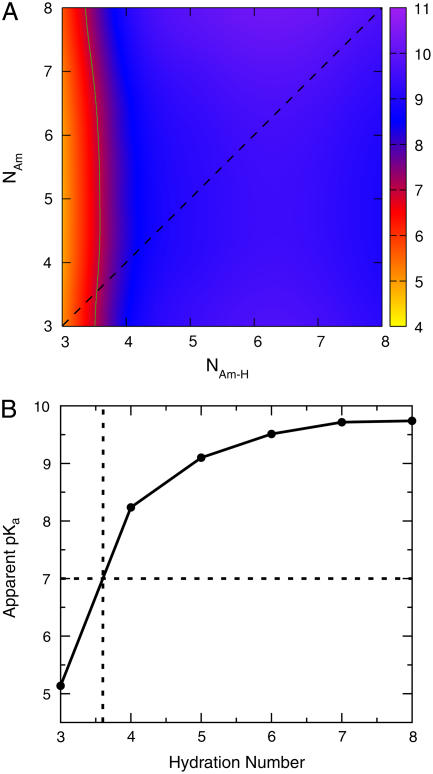
FIGURE 2.
(A) Apparent pKa of Am in a local environment that provides NAm-H hydrogen bonds for 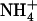 and NAm hydrogen bonds for NH3 derived from population analysis of MD trajectories for Am in dilute aqueous solution. The green contour line indicates the equivalence point (pKa = 7). The diagonal dashed line indicates where NAm-H = NAm. (B) Apparent pKa of Am for a local hydration environment where NAm-H = NAm. Note that the equivalence point occurs in an environment providing 3.6 hydrogen bonds or less (see the dotted lines).
and NAm hydrogen bonds for NH3 derived from population analysis of MD trajectories for Am in dilute aqueous solution. The green contour line indicates the equivalence point (pKa = 7). The diagonal dashed line indicates where NAm-H = NAm. (B) Apparent pKa of Am for a local hydration environment where NAm-H = NAm. Note that the equivalence point occurs in an environment providing 3.6 hydrogen bonds or less (see the dotted lines).
It is also interesting to consider this result in light of a recent study suggesting a stable water chain can enter the lumen (13) from the cytoplasm. If, indeed, this occurs, 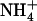 might occupy the lumen more favorably than previously thought. Our previous work (3) suggested that if
might occupy the lumen more favorably than previously thought. Our previous work (3) suggested that if 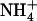 enters the lumen, it may be hydrated by as many as 2–3 water molecules (Fig. 1 B). However, the data presented here (Fig. 2) indicate Am will exist as NH3 if only three hydrogen bond partners are provided. Thus, the (de)protonation sites we suggest (Fig. 1 and Bostick and Brooks (3)) can still hold true despite a hydrated pore. Also, the preference of NH3 for threefold (or less) coordination forces us to consider the possibility that NH3 and H2O might coexist in a confined luminal environment. We suggest that future computational study aimed at determining Am's protonation state in a hydrated lumen may shed light on this issue.
enters the lumen, it may be hydrated by as many as 2–3 water molecules (Fig. 1 B). However, the data presented here (Fig. 2) indicate Am will exist as NH3 if only three hydrogen bond partners are provided. Thus, the (de)protonation sites we suggest (Fig. 1 and Bostick and Brooks (3)) can still hold true despite a hydrated pore. Also, the preference of NH3 for threefold (or less) coordination forces us to consider the possibility that NH3 and H2O might coexist in a confined luminal environment. We suggest that future computational study aimed at determining Am's protonation state in a hydrated lumen may shed light on this issue.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org
Acknowledgments
This material is based upon work supported by the National Science Foundation (NSF) under grant No. 0434578. Additional NSF support (PHYS0216576 and MCB-0413858) and support from the National Institutes of Health (RR06009) are also acknowledged.
References
- 1.Khademi, S., J. O'Connell III, J. Remis, Y. Robles-Colmenares, L. J. W. Miercke, and R. M. Stroud. 2004. Mechanism of ammonia transport by Amt/Mep/Rh structure of AmtB at 1.35 Å. Science. 305:1587–1594. [DOI] [PubMed] [Google Scholar]
- 2.Zheng, L., D. Kostrewa, Bernèche, F. K. Winkler, and X.-D. Li. 2004. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. USA. 101:17090–17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostick, D. L. and C. L. Brooks III. 2007. Deprotonation by dehydration: the origin of ammonium sensing in the AmtB channel. PLoS Comput. Biol. 3:e22/0001–0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikita, H., and E.-W. Knapp. 2007. Protonation states of ammonia/ammonium in the hydrophobic pore of ammonia transporter protein AmtB. J. Am. Chem. Soc. 129:1210–1215. [DOI] [PubMed] [Google Scholar]
- 5.Lin, Y., Z. Cao, and Y. Mo. 2006. Molecular dynamics simulations on the Escherichia coli ammonia channel protein AmtB: mechanism of ammonia/ammonium transport. J. Am. Chem. Soc. 128:10876–10884. [DOI] [PubMed] [Google Scholar]
- 6.Liu, Y., and X. Hu. 2006. Molecular determinants for binding of ammonium ion in the ammonia transporter AmtB—a quantum chemical analysis. J. Phys. Chem. A. 110:1375–1381. [DOI] [PubMed] [Google Scholar]
- 7.Nygaard, T. P., C. Rovira, G. H. Peters, and M. Ø. Jensen. 2006. Ammonium recruitment and ammonia transport by E. coli ammonia channel AmtB. Biophys. J. 91:4401–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
8.Yang, H., Y. Xu, W. Zhu, K. Chen, and H. Jiang. 2007. Detailed mechanism for AmtB conducting
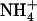 /NH3: molecular dynamics simulations. Biophys. J. 92:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
/NH3: molecular dynamics simulations. Biophys. J. 92:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar] - 9.Callebaut, I., F. Dulin, O. Bertrand, P. Ripoche, I. Mouro, Y. Colin, J.-P. Mornon, and J.-P. Cartron. 2006. Hydrophobic cluster analysis and modeling of the human Rh protein three-dimensional structures. Transfus. Clin. Biol. 13:70–84. [DOI] [PubMed] [Google Scholar]
- 10.Luzhkov, V. B., M. Almlöf, M. Nervall, and J. Åqvist. 2006. Computational study of the binding affinity and selectivity of the bacterial ammonium transporter AmtB. Biochemistry. 45:10807–10814. [DOI] [PubMed] [Google Scholar]
- 11.Javelle, A., E. Severi, J. Thornton, and M. Merrick. 2004. Ammonium sensing in Escherichia coli. J. Biol. Chem. 279:8530–8538. [DOI] [PubMed] [Google Scholar]
- 12.Bostick, D., and C. L. Brooks III. 2007. Selectivity in K+ channels is due to topological control of the permeant ion's coordinated state. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 13.Lamoureux, G., M. L. Klein, and S. Bernèche. 2007. A stable water chain in the hydrophobic pore of the AmtB ammonium transporter. Biophys. J. 92:L82–L84. [DOI] [PMC free article] [PubMed] [Google Scholar]