Abstract
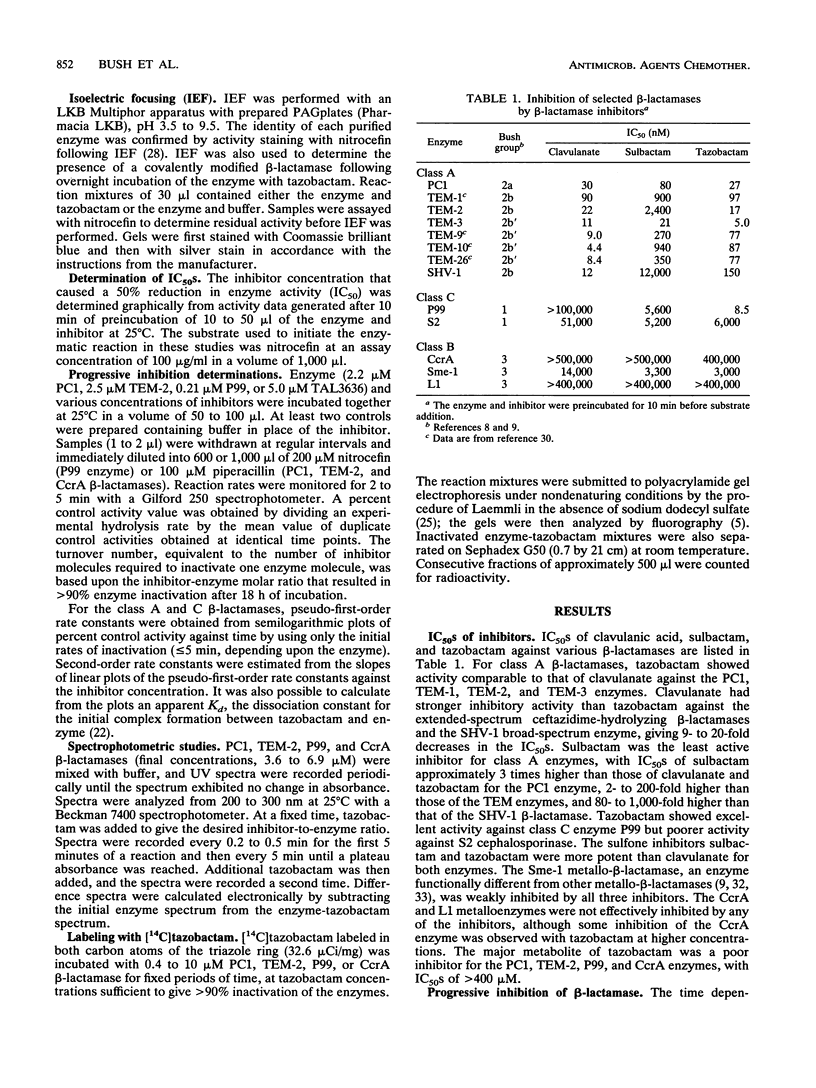
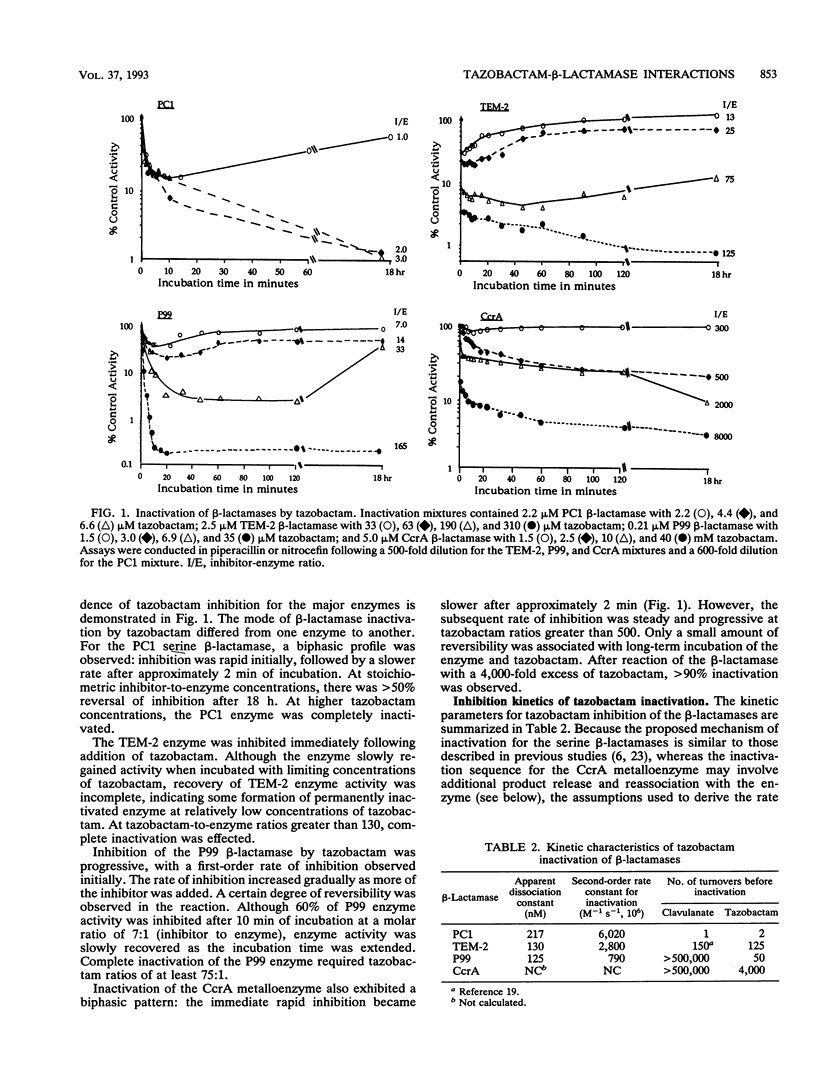
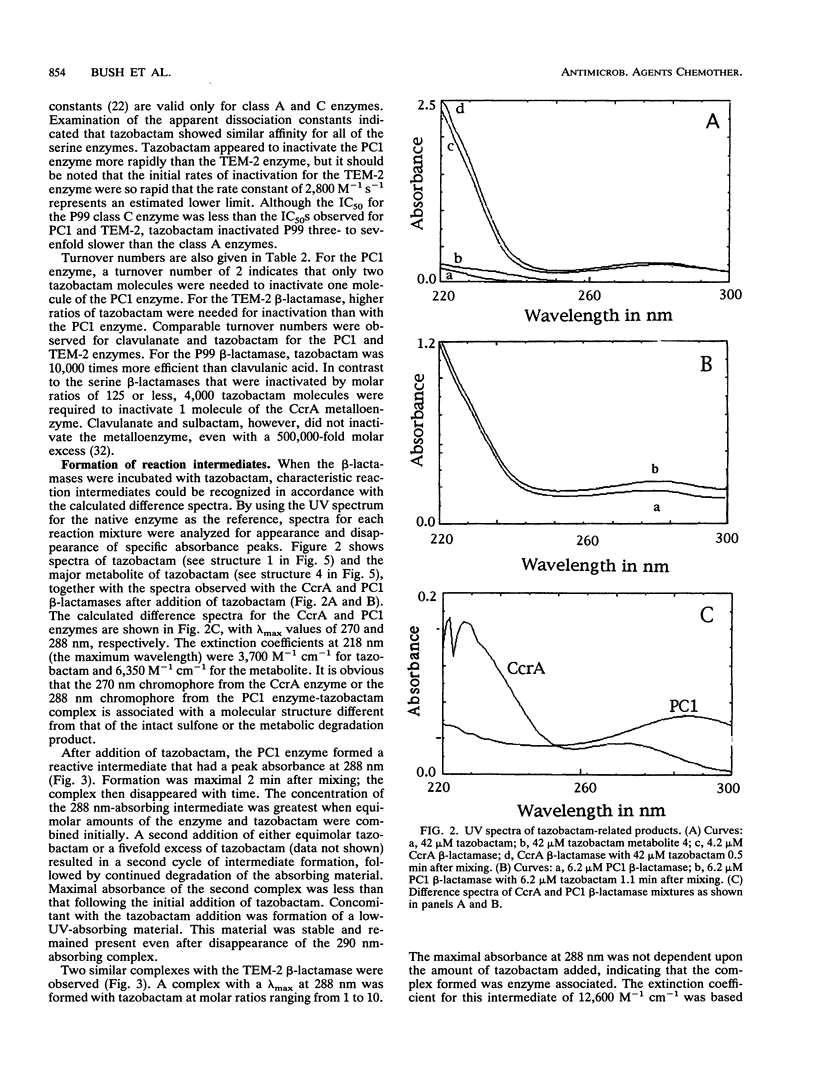
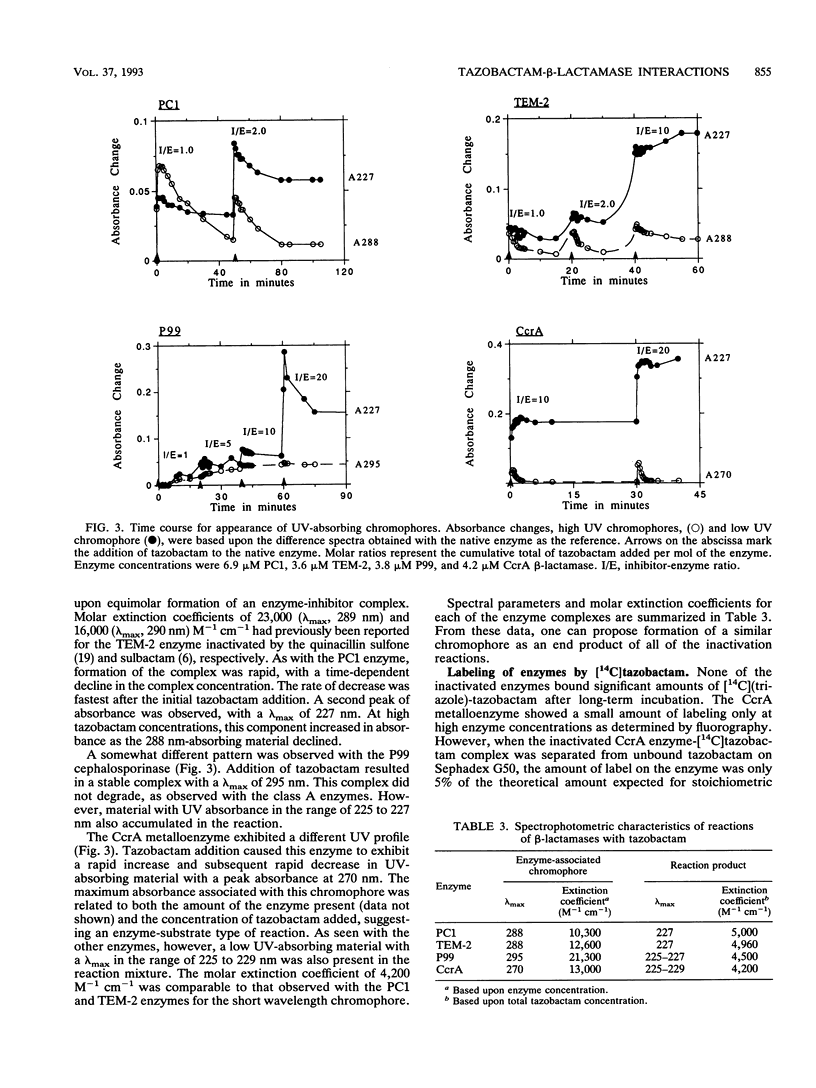
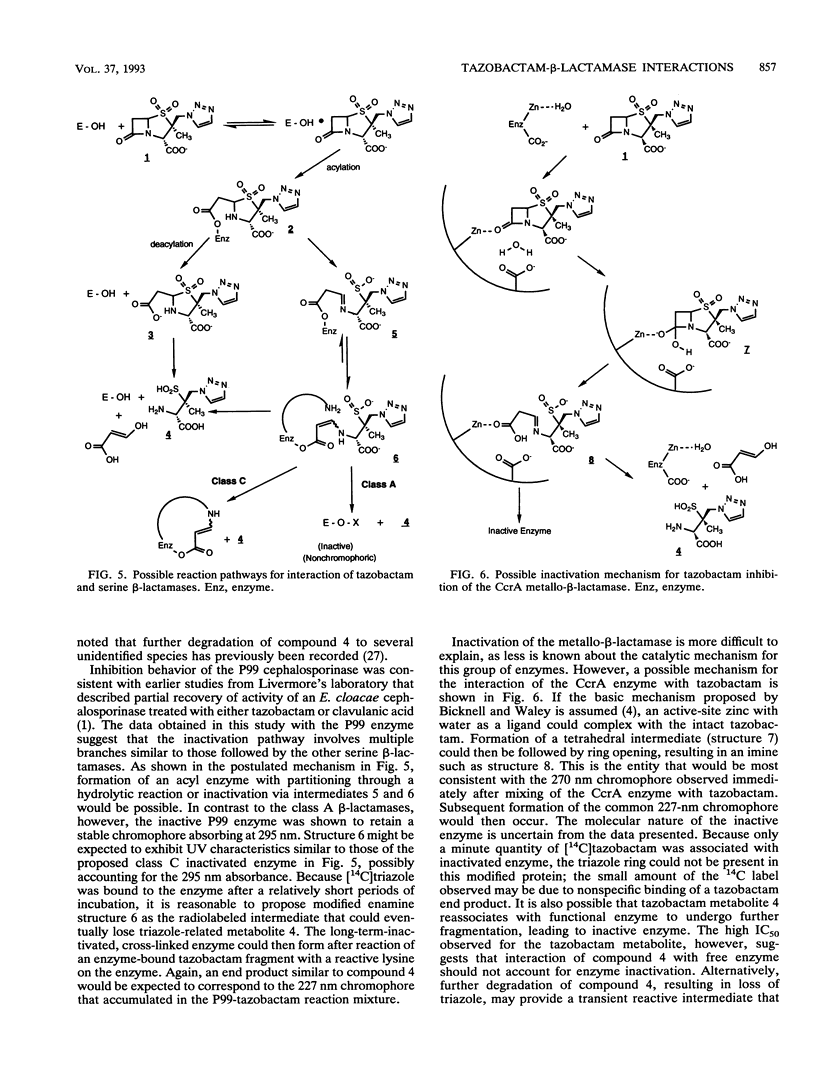
Tazobactam was shown to be a potent inhibitor of group 1, 2a, 2b, and 2b' beta-lactamases. Extended kinetic studies with class A and C serine beta-lactamases showed that the PC1, TEM-2, and P99 enzymes all were reversibly inhibited prior to inactivation of the enzymes. The CcrA metallo-beta-lactamase was less well inhibited, with a 50% inhibitory concentration at least 3 orders of magnitude less favorable than those for most serine beta-lactamases. The numbers of hydrolytic turnovers of tazobactam before inactivation were 2 for PC1, 125 for TEM-2, 50 for P99, and 4,000 for the CcrA enzyme. In spectral studies, transient intermediates were formed after reaction of tazobactam with the PC1, TEM-2, and CcrA beta-lactamases, corresponding to enzyme-associated intermediates responsible for hydrolysis of tazobactam. Chromophores absorbing at 270 nm (CcrA) and 288 nm (TEM-2 and PC1) were observed for these reaction intermediates. The P99 cephalosporinase formed a stable complex with a UV maximum at 295 nm. Incubation of tazobactam with all of the enzymes resulted in accumulation of a tazobactam reaction product with a short-wavelength absorbance. This product has characteristics similar to those of the major eucaryotic metabolite of tazobactam. Possible reaction mechanisms are presented to explain the findings. In conclusion, both serine-based and metallo-beta-lactamases were irreversibly inactivated by tazobactam following an initial transient inhibition phase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akova M., Yang Y., Livermore D. M. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed Class I beta-lactamases. J Antimicrob Chemother. 1990 Feb;25(2):199–208. doi: 10.1093/jac/25.2.199. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C., Jacobs M. R., Johenning S., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, sodium clavulanate, and sulbactam combined with amoxicillin or ampicillin. Antimicrob Agents Chemother. 1984 Oct;26(4):580–582. doi: 10.1128/aac.26.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Waley S. G. Cryoenzymology of Bacillus cereus beta-lactamase II. Biochemistry. 1985 Nov 19;24(24):6876–6887. doi: 10.1021/bi00345a021. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: an unexpected isotope effect in the interaction of 6 alpha- and 6 beta-monodeuterio and of 6,6-dideuterio derivatives with RTEM beta-lactamase from Escherichia coli. Biochemistry. 1981 Jun 23;20(13):3680–3687. doi: 10.1021/bi00516a003. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Singer S. B. Biochemical characteristics of extended broad spectrum beta-lactamases. Infection. 1989 Nov-Dec;17(6):429–433. doi: 10.1007/BF01645566. [DOI] [PubMed] [Google Scholar]
- Bush K., Singer S. B. Effective cooling allows sonication to be used for liberation of beta-lactamases from gram negative bacteria. J Antimicrob Chemother. 1989 Jul;24(1):82–84. doi: 10.1093/jac/24.1.82. [DOI] [PubMed] [Google Scholar]
- Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Coulson A. F. A semi-synthetic penicillinase inactivator. Nature. 1979 Mar 22;278(5702):360–361. doi: 10.1038/278360a0. [DOI] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnas R. L., Fisher J., Knowles J. R. Chemical studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2185–2189. doi: 10.1021/bi00604a025. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Herzberg O. Inhibition of beta-lactamase by clavulanate. Trapped intermediates in cryocrystallographic studies. J Mol Biol. 1992 Apr 20;224(4):1103–1113. doi: 10.1016/0022-2836(92)90472-v. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Prior R. B. Comparative in vitro activities of piperacillin-tazobactam and ticarcillin-clavulanate. Antimicrob Agents Chemother. 1989 Aug;33(8):1268–1274. doi: 10.1128/aac.33.8.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kemal C., Knowles J. R. Penicillanic acid sulfone: interaction with RTEM beta-lactamase from Escherichia coli at different pH values. Biochemistry. 1981 Jun 23;20(13):3688–3695. doi: 10.1021/bi00516a004. [DOI] [PubMed] [Google Scholar]
- Kuck N. A., Jacobus N. V., Petersen P. J., Weiss W. J., Testa R. T. Comparative in vitro and in vivo activities of piperacillin combined with the beta-lactamase inhibitors tazobactam, clavulanic acid, and sulbactam. Antimicrob Agents Chemother. 1989 Nov;33(11):1964–1969. doi: 10.1128/aac.33.11.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marunaka T., Maniwa M., Matsushima E., Minami Y. High-performance liquid chromatographic determination of a new beta-lactamase inhibitor and its metabolite in combination therapy with piperacillin in biological materials. J Chromatogr. 1988 Sep 23;431(1):87–101. doi: 10.1016/s0378-4347(00)83072-8. [DOI] [PubMed] [Google Scholar]
- Marunaka T., Matsushima E., Minami Y., Yoshida K., Azuma R. Degradation of beta-lactamase inhibitor, (2S,3R,5S)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-yl-methyl)-4-thi a-1- azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide (YTR-830H), in aqueous solutions and alkaline methanol solution: pathway and structural elucidation of products. Chem Pharm Bull (Tokyo) 1988 Nov;36(11):4478–4487. doi: 10.1248/cpb.36.4478. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Moosdeen F., Williams J. D., Yamabe S. Antibacterial characteristics of YTR 830, a sulfone beta-lactamase inhibitor, compared with those of clavulanic acid and sulbactam. Antimicrob Agents Chemother. 1988 Jun;32(6):925–927. doi: 10.1128/aac.32.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Quinn J. P., Miyashiro D., Patel M., Bush K., Singer S. B., Graves D., Palzkill T., Arvin A. M. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992 Sep;36(9):1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani S., Castiglia P., Maida A., Muresu E., Mezzatesta M. L., Nicoletti G. Comparative in-vitro activity of piperacillin and piperacillin plus tazobactam towards beta-lactamase producing clinical isolates. J Chemother. 1990 Oct;2(5):295–299. doi: 10.1080/1120009x.1990.11739032. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Wu P. J., Livermore D. M. Biochemical characterization of a beta-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother. 1990 May;34(5):755–758. doi: 10.1128/aac.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Rasmussen B. A., Bush K. Biochemical characterization of the metallo-beta-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992 May;36(5):1155–1157. doi: 10.1128/aac.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]