Abstract
Phytochelatin synthases (PCS) mediate cellular heavy-metal resistance in plants, fungi, and worms. However, phytochelatins (PCs) are generally considered to function as intracellular heavy-metal detoxification mechanisms, and whether long-distance transport of PCs occurs during heavy-metal detoxification remains unknown. Here, wheat TaPCS1 cDNA expression was either targeted to Arabidopsis roots with the Arabidopsis alcohol dehydrogenase (Adh) promoter (Adh::TaPCS1/cad1-3) or ectopically expressed with the cauliflower mosaic virus 35S promoter (35S::TaPCS1/cad1-3) in the PC-deficient mutant cad1-3. Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 complemented the cadmium, mercury, and arsenic sensitivities of the cad1-3 mutant. Northern blot, RT-PCR, and Western blot analyses showed Adh promoter-driven TaPCS1 expression only in roots and thus demonstrated lack of long-distance TaPCS1 mRNA and protein transport in plants. Fluorescence HPLC analyses showed that under Cd2+ stress, no PCs were detectable in cad1-3. However, in Adh::TaPCS1/cad1-3 plants, PCs were detected in roots and in rosette leaves and stems. Inductively coupled plasma atomic emission spectrometer analyses showed that either root-specific or ectopic expression of TaPCS1 significantly enhanced long-distance Cd2+ transport into stems and rosette leaves. Unexpectedly, transgenic expression of TaPCS1 reduced Cd2+ accumulation in roots compared with cad1-3. The reduced Cd2+ accumulation in roots and enhanced root-to-shoot Cd2+ transport in transgenic plants were abrogated by l-buthionine sulfoximine. The presented findings show that (i) transgenic expression of TaPCS1 suppresses the heavy-metal sensitivity of cad1-3, (ii) PCs can be transported from roots to shoots, and (iii) transgenic expression of the TaPCS1 gene increases long-distance root-to-shoot Cd2+ transport and reduces Cd2+ accumulation in roots.
Cadmium is a widespread nonessential toxic heavy metal, released into the biosphere mostly by modern industry (1). Phytochelatins (PCs) play an essential role in heavy-metal detoxification in plants and fungi (2, 3). PCs chelate heavy metals and then PC-metal complexes are translocated across the tonoplast and sequestered in vacuoles (4, 5), thus decreasing the heavy-metal content in the cytosol of yeast and plant cells. PCs are synthesized from glutathione by the enzyme PC synthase (PCS) (6–9). However, PC research has focused mainly on PC transport into vacuoles (4, 5), and it remains unknown whether long-distance transport of PCs or PCS occurs in plants.
Cd2+ is taken up by plants, and plants have been proposed to provide an efficient system for heavy-metal removal from soils (10, 11). Soil composition affects Cd2+ sensitivity. For example, silicon in soil reduces heavy-metal sensitivity (12, 13). Normally, Cd2+ concentrations in roots are at least 10 times greater than those in shoots (14). However, for efficient phytoextraction from soils, heavy metals must be translocated into aerial tissues for later harvest. Translocation of Cd2+ from roots to shoots has been studied in diverse plant species (15–18) and has been proposed to occur via the xylem of Indian mustard in a PC-independent manner (16). The molecular mechanisms for root-to-shoot Cd2+ transport remain largely unknown, and PCs are predicted not to undergo long-distance transport but to enhance vacuolar heavy-metal sequestration in the cells in which they are produced.
The cad1-3 mutant is a recessive, loss-of-function mutation in the Arabidopsis AtPCS1 gene (7) and shows no detectable PC levels under Cd2+ stress (19). In the present study, we addressed the following questions by targeted expression of the wheat TaPCS1 cDNA in the cad1-3 mutant. (i) Can PCSs or PCs be translocated from roots to shoots? (ii) Does targeted PCS expression in roots lead to Cd2+ trapping in roots, or does it enhance Cd2+ translocation and accumulation in shoots? and (iii) To what extent do PCs contribute to long-distance Cd2+ transport?
Materials and Methods
DNA Constructs and Plant Transformation. Two restriction enzyme sites, BamHI and SpeI, were introduced into the wheat TaPCS1 cDNA (9) by PCR. The TaPCS1 PCR fragment was subcloned into pBluescript II SK(+) vector. A 3× myc tag DNA sequence was constructed by PCR recovery from a plasmid containing c-myc and then subcloned into the pGEM-T Easy vector (Promega). The subcloned myc fragment was recovered with SpeI and SacI enzymes and then fused to the 3′ end of the TaPCS1 ORF in the pBluescript II SK(+) vector at the SpeI site. All PCR products were confirmed by sequencing (Retrogen, La Jolla, CA). The fusion sequence was then digested with BamHI and SacI and subcloned into the binary expression vector pBI121, either with the cauliflower mosaic virus 35S or the Arabidopsis alcohol dehydrogenase (Adh) promoter (20). Both constructs were transformed into the PC-deficient Arabidopsis mutant cad1-3 by direct Agrobacterium tumefaciens-mediated transformation using the floral dip technique (21). T2 seeds with 3:1 segregation on kanamycin plates were used for homozygote screening.
Plant Material, Growth Conditions, and Metal Stress Treatments. Seedling growth analyses were performed on one-quarter-strength Murashige and Skoog (MS) basal medium (Sigma) with 1 g/liter Mes, 0.8% agar, and the indicated concentrations of heavy metals. Homozygous transgenic plants and WT and mutant controls were grown in one-quarter-strength sterile hydroponic solution as described (22) at 24°C for a 16/8-h day/night period, with minor modifications. Plants were germinated on 0.5× MS plates with 0.6% sucrose for 2–3 days and then transferred to hydroponic solutions. The hydroponic medium was changed to refresh medium 2 days after transfer of plants and subsequently every 3–4 days. At 4 weeks of age, 20 μM CdCl2 was applied to the hydroponic solution. Root, rosette leaf, and stem samples were then harvested at timed intervals. The glutathione synthesis inhibitor l-buthionine sulfoximine (BSO) was added as indicated. Before harvesting, root samples were rinsed in deionized and filtered water for 5 min and then washed at room temperature in 25 mM CaCl2 (pH ≈5) twice, 4 min each time.
Northern Blotting and RT-PCR. Total RNA was extracted from roots, rosette leaves, and stems by using TRIzol reagent (Invitrogen). RNA gel blotting, probe labeling, and hybridizations were performed by using standard protocols suggested by the manufacturers. First-strand cDNA was synthesized from DNaseI-digested total RNA by using Maloney murine leukemia virus reverse transcriptase (Promega), and PCR was performed on PE GeneAmp 9700 with 25 cycles with Biolase TaqDNA polymerase (DocFrugal, San Diego).
Protein Extraction and Western Blotting. Plant root, rosette leaf, and stem tissues (0.25–0.5 g) were homogenized in a glass homogenizer separately, with 1 ml of extraction buffer containing 25 mM Tris·HCl (pH 7.5), 5 mM EDTA, 1 mM 2-mercaptoethanol, 1% (wt/vol) polyvinylpyrrolidone, and Complete EDTA-free (Roche Biochemicals) protease inhibitor mixture tablets (1 tablet per 50 ml of extraction buffer). Homogenates were centrifuged at 15,000 × g for 15 min to remove the debris. The protein concentration of the crude extract was determined by the Bradford method (23) with BSA as standard.
Fifteen micrograms of protein from root, stem, and rosette tissues was separated on 10% SDS/PAGE gels and electroblotted onto nitrocellulose membrane (Bio-Rad). Membranes were blocked at room temperature for 1 h in TBST (10 mM Tris·HCl, pH 7.5/150 mM NaCl/0.05% Tween 20) containing 5% dry milk and then probed with the primary mAb against the c-myc tag at a dilution of 1:400 (Sigma) at 4°C overnight. After washing, the blot was incubated with horseradish peroxidase-conjugated anti-mouse secondary Ab (Sigma) at 1:5,000 dilution in blocking buffer for 1 h. After washing again in TBST, the ECL system (Amersham Pharmacia) was used to detect the antigen–Ab complexes.
HPLC Analyses of PCs in Plant Tissues. Four-week-old plants were exposed to 20 μMCd2+ in hydroponic medium for 72 h, and then tissue extracts were prepared by using glass homogenizers and thiols labeled by monobromobimane as described (9, 24). Synthesized standards were used for the identification of PCs (γ-EC)2G (PC2), (γ-EC)3G (PC3), and (γ-EC)4G (PC4).
Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES) Analysis. Heavy-metal-treated and control plant tissues were digested in concentrated nitric acid for 5–7 days at room temperature, and then samples were boiled until completely digested. Samples were diluted with Millipore filtered deionized water and briefly centrifuged. The diluted samples were then analyzed by ICP-AES.
Results
Root-Specific and Ectopic Expression of the Wheat TaPCS1 Gene in cad1-3. The cad1-3 mutant is a recessive, loss-of-function mutation in the Arabidopsis AtPCS1 gene (7) and shows no detectable PC levels (ref. 19; see also below). Therefore, cad1-3 was selected for targeted PCS expression. To target PCS expression in roots, the Adh promoter was introduced before the wheat TaPCS1 cDNA. The wheat TaPCS1 cDNA has 49.2% nucleotide identity to the Arabidopsis gene and was used here to avoid cosilencing. The Adh promoter is highly induced in mature plant roots by hypoxia (20, 25). Therefore, a hydroponic growth system was developed in which plants can grow under hypoxic conditions. For comparison with root-targeted lines, the TaPCS1 cDNA was ectopically expressed by using the cauliflower mosaic virus 35S promoter. Independent transgenic lines for Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 with single-copy insertions were generated. Two homozygous lines expressing each construct with high levels of TaPCS1 expression were chosen for detailed analyses.
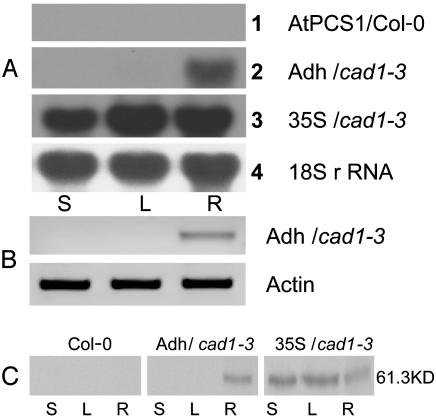
As shown in Fig. 1A, no detectable Arabidopsis AtPCS1 mRNA was observed in the WT(Col-0 ecotype) when AtPCS1 probe was hybridized to native blots. 35S::TaPCS1/cad1-3 plants grown under hydroponic conditions for 4 weeks showed transgenic expression of TaPCS1 mRNA in stems, rosette leaves, and roots (Fig. 1 A). However, in Adh::TaPCS1-transformed cad1-3 mutant plants, TaPCS1 mRNA was expressed only in roots (Fig. 1 A and B). No TaPCS1 mRNA was detectable in stems and rosette leaves of Adh::TaPCS1/cad1-3 plants even when using RT-PCR (Fig. 1B). The c-myc tag was added to the TaPCS1 cDNA in all constructs to allow analyses of TaPCS1 protein expression and determine whether the PCS protein can be translocated. Western blot analyses with anti c-myc antibodies showed root-specific expression of Adh::TaPCS1/cad1-3 and ectopic expression of 35S::TaPCS1/cad1-3 in stems, rosette leaves, and roots (Fig. 1C).
Fig. 1.
TaPCS1 mRNA and protein are not translocated from roots to shoots. (A) Northern blots probing AtPCS1 expression in WT (Col-0) (row 1) and TaPCS1 expression using the root promoter in Adh/cad1-3 (Adh::TaPCS1/cad1-3) (row 2) and the ectopic promoter in 35S/cad1-3 (35S::TaPCS1/cad1-3) (row 3). 18S rRNA was used as loading control (row 4). (B) RT-PCR was performed with TaPCS1::myc fusion-specific primers. Actin was used as control. (C) Protein was extracted from stems (S), rosette leaves (L), and roots (R) of WT (Col-0), Adh/cad1-3 (Adh::TaPCS1/cad1-3), and 35S/cad1-3 (35S::TaPCS1/cad1-3) plants. Western blot analyses were performed as described in Materials and Methods. WT protein extracts were used as negative controls.
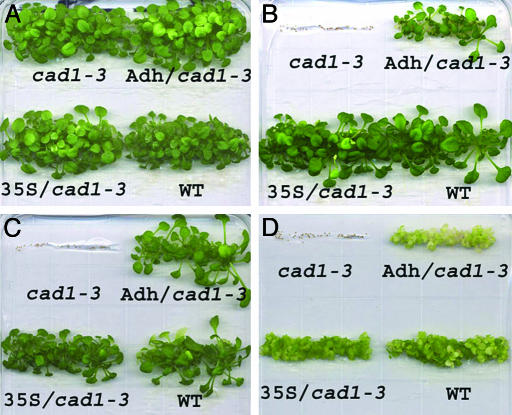
Transgenic Expression of the TaPCS1 Gene Suppresses the Heavy-Metal Sensitivity of the cad1-3 Mutant. In the absence of metal stress, seedling growth analyses showed that all plants, including the cad1-3 mutant, WT (Col-0), and transgenic plants expressing Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3, germinated and grew similar to WT plants on 0.25× Murashige and Skoog plates (Fig. 2A). When heavy metals were added to the growth medium, the cad1-3 mutant did not germinate under arsenate, mercury, and cadmium stress conditions (Fig. 2 B–D). However, WT, Adh::TaPCS1/cad1-3, and 35S::TaPCS1/cad1-3 germinated and grew in 80 μM arsenate (Fig. 2B) and 10 μM mercury (Fig. 2C). In the presence of 40 μM cadmium, Adh::TaPCS1/cad1-3 plants were slightly more sensitive to Cd2+ than WT and 35S::TaPCS1/cad1-3 seedlings (Fig. 2D). The results showed that either targeted or ectopic expression of the wheat TaPCS1 gene in the cad1-3 mutant confers tolerance to cadmium, arsenate, and mercury, but no apparent increase of tolerance was obser ved in Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 compared with WT seedlings under the imposed conditions.
Fig. 2.
TaPCS1 complements Cd2+, Hg2+, and As sensitivity in cad1-3 at seedling stage. For all panels: Upper Left, cad1-3; Upper Right, Adh/cad1-3 (Adh::TaPCS1/cad1-3); Lower Left, 35S/cad1-3 (35S::TaPCS1/cad1-3); and Lower Right, WT (Col-0). Seeds were plated and horizontally germinated on one-quarter-strength Murashige and Skoog medium with no added heavy metals (A), 80 μM KH2AsO4 (B), 10 μM HgCl2 (C), and 40 μM CdCl2 (D). Photographs were taken after 21 days.
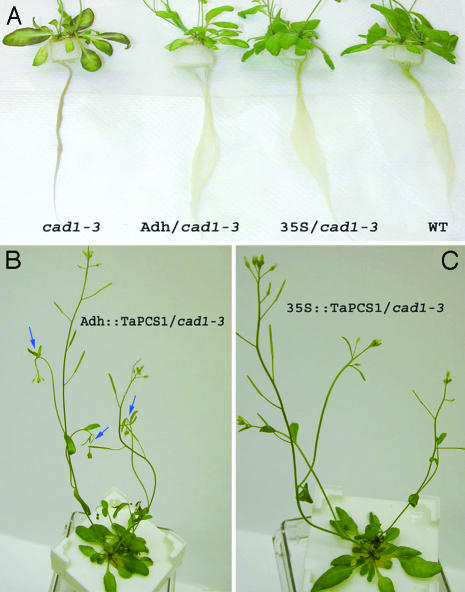
We next analyzed the effect of TaPCS1 expression in mature plants. When plants were 4 weeks old, 20 μM CdCl2 was applied to the hydroponic solution. As shown in Fig. 3A after 3 days, roots and leaves of cad1-3 plants showed brown coloration, which also has been observed for this mutant (19). In contrast, transgenic Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 plants showed root and rosette leaf growth similar to WT plants (Fig. 3A). However, further examination showed that the tissue just below the secondary shoot meristem wilted in stems of Adh::TaPCS1/cad1-3 3 days after Cd2+ addition, as indicated by arrows in Fig. 3B. In contrast, 35S::TaPCS1/cad1-3 plants exposed to 20 μM Cd2+ showed normal growth in shoot meristems (Fig. 3C). These results show that transgenic expression of TaPCS1 suppresses heavy-metal sensitivity in mature cad1-3 plants, but no significant increase of tolerance was observed compared with the WT.
Fig. 3.
Heavy-metal tolerance in cad1-3 was restored completely or partially by ectopic or root-specific expression of TaPCS1. Four-week-old hydroponically grown cad1-3 mutant, Adh/cad1-3 (Adh::TaPCS1/cad1-3), 35S/cad1-3 (35S::TaPCS1/cad1-3), and WT plants were exposed to 20 μM CdCl2 for 3 days. Phenotypes were assessed for roots and rosette leaves (A) and stems (B and C). (B) Blue arrows show sites sensitive to Cd2+ stress in Adh::TaPCS1/cad1-3.
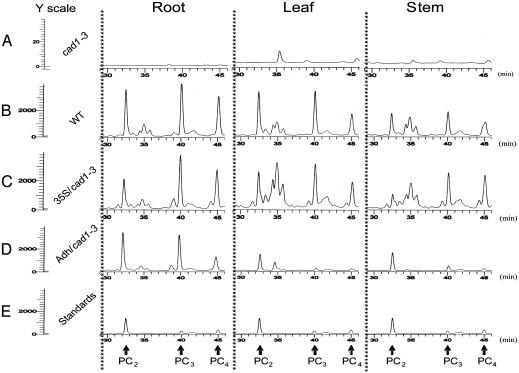
PCs Can Be Transported from Roots to Shoots. PCs have been shown to detoxify heavy metals by chelation and subsequent sequestration into vacuoles in Saccharomyces pombe and oat roots (4, 5, 26). However, it remains unknown whether PCs are transported from roots to shoots in plants and whether PCs affect long-distance metal transport. To determine whether PCs can be transported from roots to shoots, we measured PC levels in roots, rosette leaves, and stems by using fluorescence HPLC with monobromobimane as a PC-binding label. As shown in Fig. 4A, no measurable PCs were detected in roots, rosette leaves, and stems of the Arabidopsis mutant cad1-3. Note that additional peaks of unknown origin ran at 35 min (Fig. 4A), which did not correspond to PC standards (Fig. 4E). The peaks were very small in cad1-3 (note the 100-fold-magnified y-axis scale for cad1-3) (Fig. 4A). In contrast, in WT and 35S::TaPCS1/cad1-3 plants, high levels of PC2, PC3, and PC4 were detected in root, rosette leaf, and stem tissues (Fig. 4 B and C). Synthesized PC standards are shown in Fig. 4E. In Adh::TaPCS1/cad1-3, PC2, PC3, and PC4 were detected in roots at a level similar to those in WT and 35S::TaPCS1/cad1-3 roots. Interestingly, PCs were also detected in stems and rosette leaves of Adh::TaPCS1/cad1-3 (Fig. 4D), despite the finding that in Adh::TaPCS1/cad1-3, TaPCS1 protein and mRNA are root specific (Fig. 1). Both root-specific and ectopic expression of TaPCS1 did not increase PC levels significantly compared with those in the WT (Fig. 4). PC levels in rosette leaves and stems of Adh::TaPCS1/cad1-3 plants were comparable in amplitude with the PC standards (Fig. 4E). These data suggest that PCs detected in Adh::TaPCS1/cad1-3 shoots originate via translocation from roots.
Fig. 4.
PCs can be transported from roots to shoots. Four-week-old plants were exposed to 20 μM CdCl2 for 3 days in hydroponic media, and PCs were extracted from root, leaf (rosette), and stem tissues of cad1-3 (A), WT Col-0 (B), 35S/cad1-3 (35S::TaPCS1/cad1-3)(C), and Adh/cad1-3 (Adh::TaPCS1/cad1-3)(D). PC levels were measured by fluorescence HPLC. (E) Synthesized PC2, PC3, and PC4 (2 μM each) PC standards were measured, and three copies of standard measurements are illustrated for easy comparison to the measured samples. PC2, PC3, and PC4 peaks are indicated by arrows.
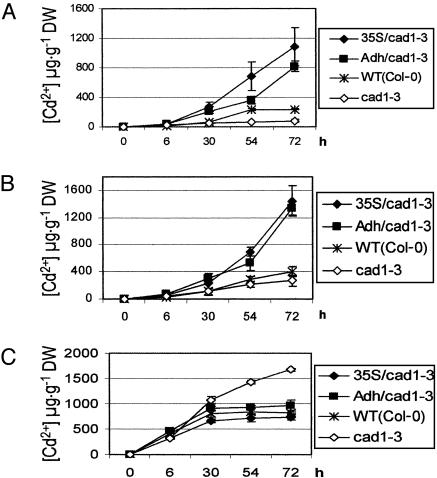
Enhancement of Long-Distance Cd2+ Transport by Transgenic Expression of TaPCS1. One of the proposed prerequisites for bioremediation is that heavy metals are transported to and sequestered in aerial parts of plants. Experiments were pursued to analyze whether targeted expression of TaPCS1 affects Cd2+ distribution in Arabidopsis. Time-course analyses of Cd2+ accumulation in stems of 4-week-old plants showed that 3 days after 20 μM Cd2+ application, very little Cd2+ was transported to stems of the cad1-3 mutant (Fig. 5A). In WT, no increase in Cd2+ accumulation was observed compared with cad1-3 at 30 h, but at 54 h and 72 h, more Cd2+ was transported to WT stems than to cad1-3 stems (Fig. 5A). Interestingly, dramatic increases in Cd2+ accumulation were observed in stems of Adh::TaPCS1/cad1-3, and 35S::TaPCS1/cad1-3 plants compared with WT and cad1-3 stems at 72 h (Fig. 5A; e.g., Adh::TaPCS1/cad1-3 vs. cad1-3, P < 0.002 and 35S::TaPCS1/cad1-3 vs. cad1-3; P < 0.021, n = 6). Furthermore, compared with cad1-3, no increase in Cd2+ accumulation was observed at 30 h in WT stems; however, in Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 stems, more Cd2+ accumulated at 30 h compared with cad1-3 (Fig. 5A; P < 0.025, n = 6). These data show that transgenic expression of TaPCS1 accelerated Cd2+ accumulation in stems. In rosette leaves (Fig. 5B), a similar Cd2+ accumulation pattern was observed in the WT and in the cad1-3 mutant. In Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3, dramatic increases in Cd2+ accumulation in rosette leaves were observed after 72 h when compared with WT and cad1-3 (Fig. 5B; e.g., Adh::TaPCS1/cad1-3 vs. cad1-3, P < 0.00017; 35S::TaPCS1/cad1-3 vs. cad1-3, P < 0.0066, n = 6). These results showed that either root-specific or ectopic expression of TaPCS1 substantially enhanced and accelerated long-distance Cd2+ transport.
Fig. 5.
Enhanced long-distance Cd2+ transport and reduced Cd2+ accumulation in roots by transgenic expression of TaPCS1. Four-week-old 35S/cad1-3 (35S::TaPCS1/cad1-3), Adh/cad1-3 (Adh::TaPCS1/cad1-3), WT (WT Col-0), and cad1-3 plants grown in hydroponic solution were exposed to 20 μM CdCl2. Samples were harvested at timed intervals after Cd2+ exposure as indicated. Cd2+ accumulation in stems (A), rosette leaves (B), and roots (C) was determined by ICP-AES. Data show mean values ± SE, n = 6 plants.
Unexpectedly, targeted expression of TaPCS1 did not enhance Cd2+ accumulation in roots (Fig. 5C). Adh::TaPCS1/cad1-3, 35S::TaPCS1/cad1-3, and WT plants showed a similar Cd2+ accumulation pattern in roots. Cd2+ accumulation was saturated 30 h after Cd2+ application. In contrast, a significant increase in Cd2+ accumulation was observed in cad1-3 roots compared with roots of Adh::TaPCS1/cad1-3, 35S::TaPCS1/cad1-3, and WT (Fig. 5C). Furthermore, Cd2+ accumulation in cad1-3 roots did not saturate within the analyzed 72-h period. Interestingly, these findings demonstrate that the presence of active PCS plays an essential role in maintaining reduced Cd2+ concentrations in Arabidopsis roots (Fig. 5C).
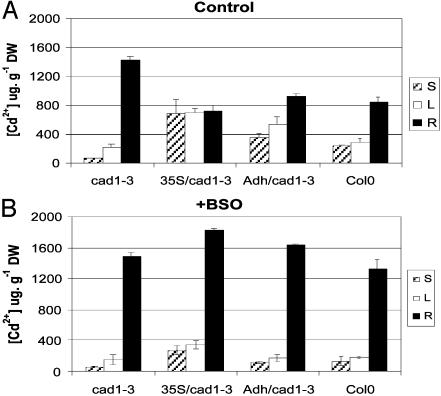
BSO Disrupts Cd2+ Homeostasis in Roots and Aborts Enhanced Long-Distance Cd2+ Transport in Transgenic TaPCS1 Plants. BSO inhibits glutathione biosynthesis and thus inhibits PC synthesis (19). Because transgenic expression of TaPCS1 enhanced long-distance Cd2+ transport (Fig. 5) but did not increase PC levels compared with WT (Fig. 4 C and D), we further analyzed whether PCs contribute to long-distance Cd2+ transport in the presence of BSO. Without BSO, WT, Adh::TaPCS1/cad1-3, and 35S::TaPCS1/cad1-3 plants accumulated more Cd2+ in stems and rosette leaves but less in roots than cad1-3 (Fig. 6A). In the presence of BSO, Cd2+ accumulation in Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 stems and rosette leaves was decreased significantly (Fig. 6B) (P < 0.03, n = 3), whereas in WT, no dramatic decrease was observed (Fig. 6B)(P > 0.22 in stems and P > 0.068 in rosette leaves, n = 3). Interestingly, addition of BSO mimicked the disruption of the AtPCS1 gene in cad1-3, resulting in increased Cd2+ accumulation in roots of Adh::TaPCS1/cad1-3, 35S::TaPCS1/cad1-3, and WT plants (Fig. 6B). BSO had no significant effect on Cd2+ accumulation in cad1-3 (Fig. 6B; P > 0.47, 0.25, and 0.67 in stems, rosette leaves, and roots, respectively; n = 3). These results demonstrate that PCs play a central role in maintaining low Cd2+ concentrations in roots and that PCs provide an essential mechanism for long-distance root-to-shoot Cd2+ transport.
Fig. 6.
Short-term BSO exposure mimics the effect of AtPCS1 gene disruption in cad1-3 with respect to Cd2+ accumulation in roots and shoots. Four-week-old cad1-3, Adh/cad1-3 (Adh::TaPCS1/cad1-3), 35S/cad1-3 (35S::TaPCS1/cad1-3), and WT plants grown in hydroponic solution were pretreated with 0 mM/1 mM BSO for 12 h; 20 μM CdCl2 was then applied to either the untreated plants (A) or the 1 mM BSO pretreated plants with 0.5 mM BSO for 54 h (B). Cd2+ accumulation in stems (S), rosette leaves (L), and roots (R) was determined by ICP-AES. Data show mean values ± SE, n = 6 and 3 plants in A and B, respectively.
Discussion
The functions of PCs in heavy-metal detoxification have been established at the intracellular and biochemical levels (2, 4, 5, 26, 27). In the present study, an important function of PCs in heavy-metal detoxification was revealed by targeting TaPCS1 to root tissues of a PC-deficient mutant, cad1-3. The presented data unexpectedly demonstrate that root-to-shoot transport of PCs occurs and that PCs provide a major mechanism for regulating long-distance Cd2+ transport in Arabidopsis.
PCs Protect Against Cd2+ Overaccumulation in Roots. PCs are known to play an essential role in heavy-metal detoxification by chelating heavy metals in the cytosol and sequestering PC-Cd2+ complexes in vacuoles via transport across the tonoplast (4, 5). Because of the important functions of PCs in cellular metal sequestration, root-specific expression of TaPCS1 has been expected to cause trapping of Cd2+ in plant roots by chelating and sequestering more Cd2+ into vacuoles.
Unexpectedly, both ectopic and root-specific expression of TaPCS1 did not increase Cd2+ content significantly in root cells (Fig. 5C). On the contrary, transgenic expression of TaPCS1 in cad1-3 caused a reduction in Cd2+ accumulation in roots compared with cad1-3. The cad1-3 mutant showed higher levels of Cd2+ accumulation in roots compared with WT and transgenic lines (Fig. 5C). Cd2+ accumulation in roots saturated in both transgenic and WT plants at 30 h after Cd2+ stress (Fig. 5C). These data show that Cd2+ accumulation in Arabidopsis roots is limited when PCs are present. Thus the PC-Cd2+ detoxification model derived from unicellular organisms (3, 4) is not completely applicable to multicellular organisms.
Consistent with the reduced Cd2+ accumulation in transgenic and WT plant roots (Fig. 5C), enhanced long-distance root-to-shoot Cd2+ transport occurred compared with cad1-3 (Fig. 5 A and B). These findings suggest that, in addition to the known cellular protection function of PCs (28), PC-dependent long-distance Cd2+ transport contributes substantially to maintenance of low Cd2+ concentrations in roots. These data further implicate a model in which Cd2+ saturation in roots causes extra Cd2+ to be transported to shoots, thus contributing to maintenance of low Cd2+ levels in roots. This proposed “overflow protection mechanism” would contribute to maintaining a low Cd2+ content in roots by transporting extra Cd2+ to shoot tissues, and the process depends on PCs (Fig. 5C).
Enhanced Long-Distance Cd2+ Transport is PC-Dependent. In cad1-3, PCs were not observed in roots, rosette leaves, and stems (Fig. 4A). However, in Adh::TaPCS1/cad1-3, PCs were found in rosette leaves and stems (Fig. 4D), although PCS protein and mRNA were detected only in roots (Fig. 1). These data show that long-distance PC transport occurs.
Interestingly, however, although much more Cd2+ was transported from roots to shoots in transgenic plants than in WT plants (Fig. 5 A and B), an increase in PC levels was not observed in transgenic plant shoots compared with WT plants (Fig. 4 B–D). Recent observations were reported showing that PC levels are generally lower in roots and slightly higher in shoots of the Zn/Cd hyperaccumulator Thlaspi caerulescens than in the non-accumulator Thlaspi arvense, despite the higher cadmium concentration in the hyperaccumulator (29). Interestingly, the present study shows that whole-tissue PC levels may not always be a good indicator of the roles of PCs in metal accumulation. Our data show that targeted PC synthesis rather than total tissue PC levels is important for Cd accumulation in Arabidopsis shoots. Surprisingly, in stems and rosette leaves of Adh::TaPCS1/cad1-3 plants, lower PC levels were detected than in the WT. This finding may be partially attributed to the fact that PCs in Adh::TaPCS1/cad1-3 originated from roots, whereas in 35S::TaPCS1/cad1-3 and WT, PCs synthesis also occurred in shoots. Short-term pharmacological experiments were pursued by using BSO, a specific inhibitor of glutathione biosynthesis, which is required for PC biosynthesis. These experiments demonstrated that BSO significantly decreased Cd2+ accumulation in shoots and enhanced Cd2+ levels in roots of Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 plants, which further supports that the enhanced root-to-shoot Cd2+ transport in transgenic plants is PC-dependent.
Possible Mechanisms Regulating Long-Distance Cd2+ Transport. Our findings point to the question of why only the exogenous expression of TaPCS1 significantly enhanced root-to-shoot Cd2+ transport despite the fact that WT plants also synthesize PCs in roots. One possible answer is that transgenic expression of TaPCS1 may cause increased PCS accumulation in specific cell types such as vascular parenchyma cells, thus allowing more PC accumulation in these cells, which could further improve Cd2+ or PC-Cd2+ loading into vascular transport pathways. In addition, recombinant PCS protein alone is sufficient to encode for the enzymatic activity required for PC synthesis. As the transgenic wheat TaPCS1 protein differs in amino acid sequence (55% amino acid identity) from the native Arabidopsis AtPCS1 protein, it is conceivable that the transgenic protein acts constitutively and more independently from a possible regulatory network in Arabidopsis. The accelerated Cd2+ accumulation in Adh::TaPCS1/cad1-3 and 35S::TaPCS1/cad1-3 stems (Fig. 5A) supports this hypothesis. Similarly, a recent study has shown that transgenic altered subcellular targeting of methylmercury lyase enhances mercury detoxification in Arabidopsis (30). Further research using cell-specific promoters will be needed to determine important sites of PC synthesis for long-distance transport.
An alternative (nonexclusive to the above) model is also possible, in which the excessive PCS mRNA in transgenic plant roots before Cd2+ stress (Fig. 1 A) may cause signal transduction events, reporting that plants have been challenged by severe Cd2+ concentrations, thus leading to up-regulation of long-distance Cd2+ transport mechanisms. These long-distance transport mechanisms could contribute to the overflow protection machinery.
In Brassica juncea WT plants, Cd2+ transport in the xylem sap was reported to be coordinated with oxygen or nitrogen ligands (16). In transgenic Arabidopsis plants, the presented data suggest that PC-assisted Cd2+ transport also occurs. The finding that the enhanced root-to-shoot Cd2+ transport was aborted by BSO addition in transgenic plants (Fig. 6) supports this model. Based on our PC standards (Fig. 4E) and the amplitude of PC2, PC3, and PC4 peaks in rosette leaves and stems of Adh::TaPCS1/cad1-3 plants, we roughly estimate that the stoichiometry of Cd2+/total PCs ranged approximately from 2.4:1 to 4.4:1. Recent research has suggested that Cd2+ binds to PC3 with a stoichiometry of 3 Cd2+/1 PC3 in oat roots (5). This simple estimate suggests that PCs function as important components of root-to-shoot cadmium transport in Adh::TaPCS1/cad1-3 plants. Note, however, that the present study does not exclude that PCs may function parallel to or contribute to other long-distance Cd2+ transport mechanisms. As suggested by the cadmium-induced secondary meristem wilting phenotype in mature Adh::TaPCS1/cad1-3 plants (Fig. 3B), Cd2+ is slightly toxic in the stems of Adh::TaPCS1/cad1-3. These observations suggest the existence of less-detoxifying long-distance Cd2+ transport mechanisms. Furthermore, these observations suggest a function for local PC synthesis in stems for Cd2+ detoxification.
In conclusion, by developing a targeted expression system, we demonstrate that PCs can be transported from roots to shoots and that PCs play an essential role in maintaining reduced Cd2+ accumulation levels in roots and enhancing long-distance root-to-shoot Cd2+ transport.
Acknowledgments
We thank Dr. Christopher Cobbett (University of Melbourne, Australia) for providing cad1-3 seeds, Kevin Walda (Scripps Institute of Oceanography, University of California, San Diego) and Dr. Jeffery Harper (The Scripps Research Institute, La Jolla, CA) for use of ICP-AES machines, Dr. Robert Fahey (University of California at San Diego) for use of HPLC equipment, Dr. Izumi Mori for help with protein extraction, and David Waner and Alice Chen for careful reading of the manuscript. This research was supported by National Institute on Environmental Health Sciences Superfund Grant 1 P42 ESI0337 and in part by National Science Foundation Grant DBI-0077378 (to J.I.S.) and Environmental Protection Agency STAR Fellowship U-91582701-1 (to D.A.L.).
Abbreviations: Adh, Arabidopsis alcohol dehydrogenase; PC, phytochelatin; PCS, PC synthase; BSO, l-buthionine sulfoximine; ICP-AES, inductively coupled plasma atomic emission spectrometer.
References
- 1.Ayres, R. U. (1992) Proc. Natl. Acad. Sci. USA 89, 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grill, E., Winnacker, E.-L. & Zenk, M. H. (1985) Science 230, 674–676. [DOI] [PubMed] [Google Scholar]
- 3.Mutoh, N. & Hayashi, Y. (1988) Biochem. Biophys. Res. Commun. 151, 32–39. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz, D. F., Ruscitti, T., McCue, K. F. & Ow, D. W. (1995) J. Biol. Chem. 270, 4721–4728. [DOI] [PubMed] [Google Scholar]
- 5.Salt, D. E. & Rauser, W. E. (1995) Plant Physiol. 107, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grill, E., Löffler, S., Winnacker, E.-L. & Zenk, M. H. (1989) Proc. Natl. Acad. Sci. USA 86, 6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha, S.-B., Smith, A. P., Howden, R., Dietrich, W. M., Bugg, S., O'Connell, M. J., Goldsbrough, P. B. & Cobbett, C. S. (1999) Plant Cell 11, 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vatamaniuk, O. K., Mari, S., Lu, Y.-P. & Rea, P. A. (1999) Proc. Natl. Acad. Sci. USA 96, 7110–7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens, S., Kim, E. J., Neumann, D. & Schroeder, J. I. (1999) EMBO J. 18, 3325–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskin, I., Kumar, N., Dushenkov, S. & Salt, D. E. (1994) Curr. Opin. Biotechnol. 5, 285–290. [Google Scholar]
- 11.Raskin, I. (1996) Proc. Natl. Acad. Sci. USA 93, 3164–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein, E. (1999) Annu. Rev Plant Physiol. Plant Mol. Biol. 50, 641–664. [DOI] [PubMed] [Google Scholar]
- 13.Epstein, E. (1994) Proc. Natl. Acad. Sci. USA 91, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaney, R. L., Malik, M., Li, Y. M., Brown, S. L., Brewer, E. P., Angle, J. S. & Baker, A. J. (1997) Curr. Opin. Biotechnol. 8, 279–284. [DOI] [PubMed] [Google Scholar]
- 15.Yang, X., Baligar, V. C., Martens, D. C. & Clark, R. B. (1995) J. Environ. Sci. Health B 30, 569–583. [Google Scholar]
- 16.Salt, D. E., Prince, R. C., Pickering, I. J. & Raskin, I. (1995) Plant Physiol. 109, 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalil, A., Selles, F. & Clarke, J. M. (1994) J. Plant Nutr. 17, 1839–1858. [Google Scholar]
- 18.Florijn, P. J., Knecht, J. A. & Van Beusichem, M. L. (1993) J. Plant Physiol. 142, 537–542. [Google Scholar]
- 19.Howden, R., Goldsbrough, P. B., Andersen, C. R. & Cobbett, C. S. (1995) Plant Physiol. 107, 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKendree, W. L., Jr., & Ferl, R. J. (1992) Plant Mol. Biol. 19, 859–862. [DOI] [PubMed] [Google Scholar]
- 21.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- 22.Arteca, R. N. & Arteca, J. M. (2000) Physiol. Plant. 108, 188–193. [Google Scholar]
- 23.Bradford, M. M. (1976) Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 24.Sneller, F. E., van Heerwaarden, L. M., Koevoets, P. L., Vooijs, R., Schat, H. & Verkleij, J. A. (2000) J. Agric. Food Chem. 48, 4014–4019. [DOI] [PubMed] [Google Scholar]
- 25.Chung, H.-J. & Ferl, R. J. (1999) Plant Physiol. 121, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz, D. F., Kreppel, L., Speiser, D. M., Schreel, G., McDonald, G. & Ow, D. W. (1992) EMBO J. 11, 3491–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grill, E. (1987) Experientia 52, Suppl., 317–322. [DOI] [PubMed] [Google Scholar]
- 28.Zenk, M. H. (1996) Gene 179, 21–30. [DOI] [PubMed] [Google Scholar]
- 29.Ebbs, S., Lau, I., Ahner, B. & Kochian, L. (2002) Planta 214, 635–640. [DOI] [PubMed] [Google Scholar]
- 30.Bizily, S. P., Kim, T., Kandasamy, M. K. & Meager, R. B. (2003) Plant Physiol. 131, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]