Abstract
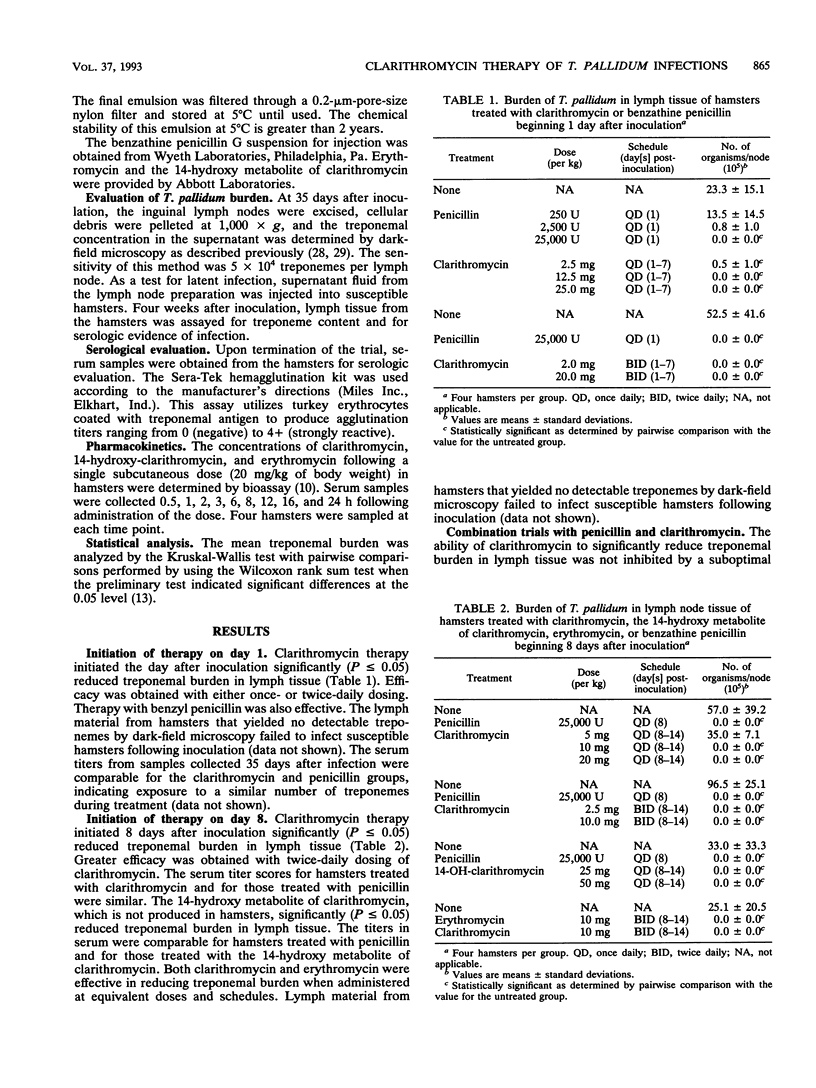
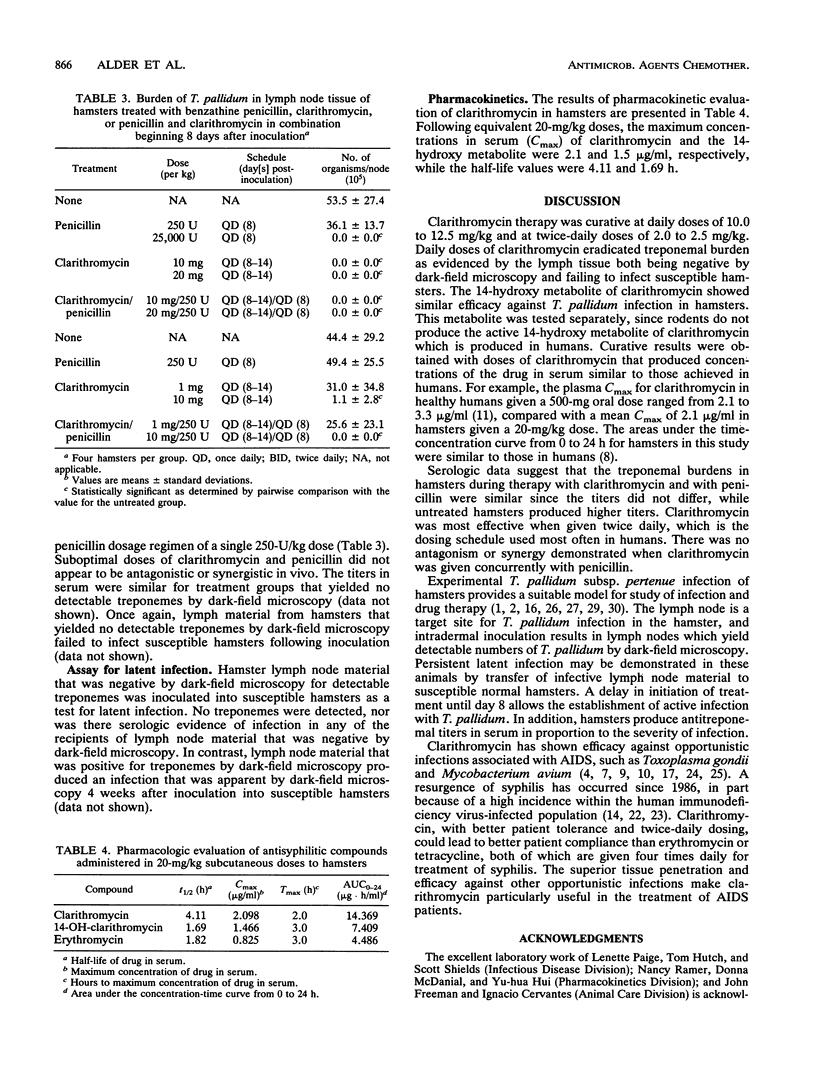
Clarithromycin was shown to be effective therapy for Treponema pallidum infections in hamsters. Clarithromycin therapy was effective when initiated either 1 or 8 days after infection. The delay in initiation of therapy allowed an active infection to develop. The treponemal burden in lymph tissue of treated hamsters was eradicated, as determined by dark-field microscopy and by inoculation of lymph material into susceptible hamsters. Treatments with clarithromycin and the 14-hydroxy metabolite of clarithromycin were equally effective. Therapy with clarithromycin and penicillin was not antagonistic and did not appear to be synergistic when the two drugs were given concurrently. Pharmacokinetic evaluation of clarithromycin in hamsters showed that the doses which produced effective therapy yielded concentrations in serum similar to those routinely achieved in human sera. These findings demonstrate that clarithromycin is effective in treating active or incubating syphilis in the hamster model and could be useful in treating humans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Damjanov I. Ability of macrophages to process and present Treponema pallidum Bosnia A strain antigens in experimental syphilis of syrian hamsters. Infect Immun. 1982 Apr;36(1):176–183. doi: 10.1128/iai.36.1.176-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Kushner H., Hashemi S. Lymphocyte function in experimental endemic syphilis of Syrian hamsters. Immunology. 1985 Sep;56(1):9–21. [PMC free article] [PubMed] [Google Scholar]
- Bayne L. L., Schmidley J. W., Goodin D. S. Acute syphilitic meningitis. Its occurrence after clinical and serologic cure of secondary syphilis with penicillin G. Arch Neurol. 1986 Feb;43(2):137–138. doi: 10.1001/archneur.1986.00520020031012. [DOI] [PubMed] [Google Scholar]
- Berlin O. G., Young L. S., Floyd-Reising S. A., Bruckner D. A. Comparative in vitro activity of the new macrolide A-56268 against mycobacteria. Eur J Clin Microbiol. 1987 Aug;6(4):486–487. doi: 10.1007/BF02013117. [DOI] [PubMed] [Google Scholar]
- Berry C. D., Hooton T. M., Collier A. C., Lukehart S. A. Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med. 1987 Jun 18;316(25):1587–1589. doi: 10.1056/NEJM198706183162507. [DOI] [PubMed] [Google Scholar]
- Chang H. R., Rudareanu F. C., Pechère J. C. Activity of A-56268 (TE-031), a new macrolide, against Toxoplasma gondii in mice. J Antimicrob Chemother. 1988 Sep;22(3):359–361. doi: 10.1093/jac/22.3.359. [DOI] [PubMed] [Google Scholar]
- Chu S. Y., Deaton R., Cavanaugh J. Absolute bioavailability of clarithromycin after oral administration in humans. Antimicrob Agents Chemother. 1992 May;36(5):1147–1150. doi: 10.1128/aac.36.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Ramer N., Rode R. A., Freiberg L. Bioassay for A-56268 (TE-031) and identification of its major metabolite, 14-hydroxy-6-O-methyl erythromycin. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):73–76. doi: 10.1007/BF01962181. [DOI] [PubMed] [Google Scholar]
- Greene B. M., Miller N. R., Bynum T. E. Failure of penicillin G benzathine in the treatment of neurosyphilis. Arch Intern Med. 1980 Aug;140(8):1117–1118. [PubMed] [Google Scholar]
- Hardy D. J., Guay D. R., Jones R. N. Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological, and clinical overview. Diagn Microbiol Infect Dis. 1992 Jan;15(1):39–53. doi: 10.1016/0732-8893(92)90055-x. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd Syphilis and HIV infection. J Infect Dis. 1989 Sep;160(3):530–534. doi: 10.1093/infdis/160.3.530. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Tierney M., Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987 Jun 18;316(25):1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- Kajdacsy-Balla A., Howeedy A., Bagasra O. Syphilis in the Syrian hamster. A model of human venereal and congenital syphilis. Am J Pathol. 1987 Mar;126(3):599–601. [PMC free article] [PubMed] [Google Scholar]
- Khardori N., Rolston K., Rosenbaum B., Hayat S., Bodey G. P. Comparative in-vitro activity of twenty antimicrobial agents against clinical isolates of Mycobacterium avium complex. J Antimicrob Chemother. 1989 Nov;24(5):667–673. doi: 10.1093/jac/24.5.667. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A. Roxithromycin (RU 965): effective therapy for experimental syphilis infection in rabbits. Antimicrob Agents Chemother. 1987 Feb;31(2):187–190. doi: 10.1128/aac.31.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz D. M., Beutner K. R., Maggio R. P., Reichman R. C. Failure of recommended treatment for secondary syphilis. JAMA. 1986 Apr 4;255(13):1767–1768. [PubMed] [Google Scholar]
- Moskovitz B. L., Klimek J. J., Goldman R. L., Fiumara N. J., Quintiliani R. Meningovascular syphilis after 'appropriate' treatment of primary syphilis. Arch Intern Med. 1982 Jan;142(1):139–140. [PubMed] [Google Scholar]
- Musher D. M., Hamill R. J., Baughn R. E. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990 Dec 1;113(11):872–881. doi: 10.7326/0003-4819-113-11-872. [DOI] [PubMed] [Google Scholar]
- Musher D. M. Syphilis, neurosyphilis, penicillin, and AIDS. J Infect Dis. 1991 Jun;163(6):1201–1206. doi: 10.1093/infdis/163.6.1201. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Mar;35(3):462–470. doi: 10.1128/aac.35.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., LeFrock J. L., Bagasra O. Endemic syphilis: transfer of resistance to Treponema pallidum strain Bosnia A in hamsters with a cell suspension enriched in thymus-derived cells. J Infect Dis. 1980 Jun;141(6):752–758. doi: 10.1093/infdis/141.6.752. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Le Frock J. L., Babu J. P., Chan J. K. Use of CB hamsters in the study of Treponema pertenue. Br J Vener Dis. 1979 Oct;55(5):316–319. doi: 10.1136/sti.55.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection and transfer of resistance with immune T cells. Adv Exp Med Biol. 1981;134:291–300. doi: 10.1007/978-1-4757-0495-2_26. [DOI] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection. Infect Immun. 1980 Jun;28(3):909–913. doi: 10.1128/iai.28.3.909-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor D. R., Bagasra O., Jacobs R. F. Treponemal infection specifically enhances node T-cell regulation of macrophage activity. Infect Immun. 1986 Oct;54(1):21–27. doi: 10.1128/iai.54.1.21-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



