Abstract
It is now well established that subthalamic nucleus high-frequency stimulation (STN HFS) alleviates motor problems in Parkinson's disease. However, its efficacy for cognitive function remains a matter of debate. The aim of this study was to assess the effects of STN HFS in rats performing a visual attentional task. Bilateral STN HFS was applied in intact and in bilaterally dopamine (DA)-depleted rats. In all animals, STN HFS had a transient debilitating effect on all the variables measured in the task. In DA-depleted rats, STN HFS did not alleviate the deficits induced by the DA lesion such as omissions and latency to make correct responses, but induced perseverative approaches to the food magazine, an indicator of enhanced motivation. In sham-operated controls, STN HFS significantly reduced accuracy and induced perseverative behaviour, mimicking partially the effects of bilateral STN lesions in the same task. These results are in line with the hypothesis that STN HFS only partially mimics inactivation of STN produced by lesioning and confirm the motivational exacerbation induced by STN inactivation.
Keywords: basal ganglia, cognitive functions, deep brain stimulation, dopamine
Introduction
Striatal dopamine depletion, the hallmark of Parkinson's disease (PD), is associated with an abnormal activity of the subthalamic nucleus (STN) (Robledo & Feger, 1991). Inactivation of the STN has thus been proposed as an alternative therapy to dopaminergic treatments in Parkinsonism (Bergman et al., 1990). STN lesions alleviate motor deficits observed in monkeys and rodent models of PD (Bergman et al., 1990; Aziz et al., 1991; Baunez et al., 1995; Henderson et al., 1999; Phillips & Brown, 1999), while in patients, accidental infarcts of the STN or surgical lesions significantly reduce parkinsonian symptoms (Sellal et al., 1992; Gill & Heywood, 1997). In PD, therapeutic lesions of the STN, a highly vascularized and small structure, may produce hemiballism and morbidity, however. Neurosurgeons have therefore developed high-frequency stimulation (HFS), a technique that was found greatly to alleviate parkinsonian motor symptoms when applied to the STN (Limousin et al., 1995). Beneficial effects are also observed on muscular rigidity and resting tremor in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys (Benazzouz et al., 1993; Gao et al., 1999). The effects of STN HFS on cognitive function remain, however, poorly documented (Benabid et al., 2001; Hershey et al., 2004; Witt et al., 2004, 2006). Understanding the mode of action of STN HFS is a goal of current research. In anesthetized rats, STN HFS is followed by a decrease in neuronal activity in the STN target structures (e.g. substantia nigra pars reticulata and entopeduncular nucleus) (Benazzouz et al., 1995; Benazzouz et al., 2000), and in 6-hydroxydopamine (6-OHDA) lesioned rats, STN HFS normalizes the increased expression of the metabolic marker in these structures (Salin et al., 2002). STN HFS is therefore thought to inactivate the STN by reducing the overactive outflow in basal ganglia outputs. However, electrophysiological and microdialysis evidence suggests that STN HFS effects arise not simply from an inactivation of STN, but also an activation of GABAergic fibres in the STN (Windels et al., 2000; Garcia et al., 2003, 2005; Degos et al., 2005; Meissner et al., 2005). Most of these data, except those from the study by Degos et al. (2005), have been obtained in rats or slices with stimulation parameters that were not proven to have beneficial effects in awake animals. STN HFS administered to behaving rats has been studied so far in either basis motor paradigms (Chang et al., 2003; Darbaky et al., 2003) or in choice reaction time tasks (Darbaky et al., 2003; Desbonnet et al., 2004; Temel et al., 2005) with a beneficial effect of STN HFS on motor functions, but only a limited effect on the ‘cognitive’ functions recruited in the performance of a choice reaction time task (Darbaky et al., 2003; Temel et al., 2005). However, to date, no study has investigated the effects of STN HFS in intact and parkinsonian animals on attentional performance, although experiments in rats have shown an involvement of STN in attentional and motivational functions (Baunez & Robbins, 1997, 1999a,b; Baunez et al., 2002, 2005). Therefore, the aim of the present study was to investigate the behavioural consequences of STN HFS in both intact and dopamine (DA)-depleted rats trained to perform a test of sustained and divided visual attention, the five-choice serial reaction time task (Robbins, 2002). In this task, rats are trained to detect brief visual stimuli presented randomly in an array. Performance measures include response accuracy and latency to make a correct response (i.e. correct latency), as well as measures of inhibitory response control (such as premature responses, perseverative responses) and motivational factors (perseverative approaches to the magazine). Adequate performance of the task depends in particular on fronto-striatal systems (Christakou et al., 2001). We have previously shown that performance is susceptible to excitotoxic or DA-depleting lesions of the striatum, inducing a slight decreased accuracy, and increased latencies, number of omissions and perseverative responses (Baunez & Robbins, 1999a; Rogers et al., 2001). In this study, we used 6-OHDA to deplete striatal DA to mimic some of the neuropathological symptoms of PD, and then employed STN HFS to restore performance.
Materials and methods
Animals
Male Lister Hooded rats (Charles River, UK) were housed in pairs and maintained on a 12-h light/dark cycle. They were kept at 85% of their free feeding weight by food restriction. Water was provided ad libitum. All experimental procedures were subject to UK Home Office approval (Project Licence PPL 80/1324).
Surgery
All animals were anaesthetized with xylazine (7.5 mg/kg, i.m.) and ketamine (50 mg/kg, i.m.) and secured in a Kopf stereotaxic apparatus. Rats received a bilateral injection of 6-OHDA (Sigma, UK; 12 μg/3 μL) (n = 32) or vehicle (ascorbate, 0.1 mg/mL) (n = 30) delivered by a micropump (Harvard Apparatus Ltd, Kent, UK) in the dorsal striatum at the following coordinates AP: +0.2 mm, L: ± 3.5 mm (from bregma), DV: −4.0 mm (from skull) (Paxinos & Watson, 1986). Selected animals were further implanted bilaterally with a platinium electrode into the STN (AP: −3.8 mm, L: ± 2.4 mm, DV: −8.35 mm). As described previously (Darbaky et al., 2003) the electrode was formed by two platinium wires (Phymep, Paris, France) insulated with Teflon (diameter 110 μm each) and inserted in a stainless steel tubing leading to an average distance of 100 μm between the tips. The Teflon on the protruding tip of both wires was removed over 2 mm (diameter 76 μm). One week of recovery was allowed before behavioural testing.
High-frequency stimulation of the STN
The electrical parameters for the stimulation were chosen according to those determined in an earlier behavioural study showing reversal of basic motor deficits induced by DA depletion (frequency, 130 Hz; pulse width, 60 μs; intensity, 50 μA; Darbaky et al., 2003). The stimulation was applied just before the session and remained on throughout the daily 30-min session.
Apparatus
The test apparatus consisted of four 25 × 25-cm aluminium chambers (Cambridge Cognition, Cambridge, UK). The rear wall of each chamber was concavely curved and contained nine apertures, each 2.5 cm square, 4 cm deep and set 2 cm above floor level. Illumination of each hole was provided by a standard 3-W bulb located at the rear of the hole. In addition, located at the entrance of each aperture was an infrared photocell beam monitoring the nose poke responding of the rat. Each aperture could be blocked by a metal cover when not required and for the present task apertures 1, 3, 5, 7 and 9 were open (for details see Carli et al., 1983).
The four chambers were individually housed within sound-attenuating cabinets and were ventilated by low-level noise fans, which also served to mask extraneous background noise. The apparatus and on-line data collection was controlled by means of an Acorn computer system with software written by Dr R. N. Cardinal.
Five-choice serial reaction time task procedure
Rats were trained to discriminate a brief visual stimulus presented randomly in one of the 5 spatial locations (hole number 1, 3, 5, 7, 9), as described previously (e.g. Muir et al., 1996; Baunez & Robbins, 1997). At the beginning of each test session, the house light was illuminated and free delivery of a single food pellet to the magazine was made. The trial was initiated by the rat opening the panel to collect this pellet. After a fixed 5-s intertrial interval (ITI), the light at the rear of one of the apertures was illuminated for a short period (0.5 s). Responses in this aperture during illumination and for 5 s afterwards (the limited hold period) were rewarded with the delivery of a food pellet and a correct response was recorded. Responses in a non-illuminated hole during the signal period (incorrect response) and failures to respond within the limited hold period (omission) were punished with a period of darkness during which all lights were extinguished for 5 s (time out). Responses in the apertures during the ITI were recorded as premature responses and were punished with time out. Additional responses in the apertures during the limited hold period were recorded as perseverative responses. A response in the food panel after the delivery of a food pellet, or after the time-out period, initiated the next trial.
During each session, the light stimulus was presented an equal number of times in each of the five apertures in a random order. A daily session consisted of 100 trials or was terminated after 30 min of testing. For the first session of training, the stimulus duration and limited hold periods were both set at 1 min, and the ITI and time-out periods set at 5 s. These variables were altered on subsequent sessions according to the individual animal's performance, until the target set of task parameters could be instituted. The target parameters were: stimulus duration, 0.5 s; limited hold period, 5 s; ITI and time-out period, 5 s. The animals were considered to have reached the criterion when these target parameters were attained on five consecutive sessions with > 80% correct responses and < 20% omissions within the 30-min session time. Approximately 30 sessions were required for the animals to attain this criterion.
Two weeks after surgery, the animals used in this experiment were tested across six sessions on the standard schedule of the task to characterize the effects of the DA depletion (block Post). Then, the effects of STN HFS were assessed for 12 consecutive sessions (two blocks of six sessions: stim1 and stim2). An extra set of six daily sessions with stimulation off was run at the end of the experiment (block stim OFF).
Experimental procedure
The lesion group was divided into two subgroups (6-OHDA, n = 16; 6-OHDA-HFS, n = 16). The same subdivision was applied in the sham group (sham, n = 14; sham-HFS, n = 16).
Statistical analysis
The results are expressed as means for each of the variables (i.e. accuracy, omissions, premature responses, perseverative responses, perseverative panel pushes per session) in the different groups of animals. For each block of six daily sessions, the average was calculated and the mean per session per rat was considered for statistical analysis. The data were analysed using various designs of anova (Statview ver. 5.0, Abacus concepts, Berkeley, CA, USA), as follows. For each variable of the task, the data were submitted first to a global mixed-design anova with Group (sham vs. sham-HFS vs. 6-OHDA vs. 6-OHDA-HFS) as the between-subject factors and with the various blocks of sessions as the within-subject factor. One-way anova was then performed when significant effects and interactions were found after the global anova. When significant effects were found, post-hoc comparisons between means were further performed as appropriate.
Histology
After completion of the behavioural testing, all the animals were killed by decapitation. The fresh brains were removed and frozen in dry-ice to be kept at −80 °C. Frontal 10-μm-thick sections of the STN were cut and collected for cresyl violet staining. Striatal sections were incubated with 50 nm [3H]mazindol (17 Ci/mm, 0.3 mm desipramine; NEN DuPont, Zaventem, Belgium) to assess the depletion in DA terminals within the striatum, following the protocol described by Javitch et al. (1985) and routinely used in the laboratory (Hajji et al., 1996). Autoradiographs were generated by apposing the sections on a 3H-sensitive screen (Raytest, Paris, France) for 7 days and were further quantified by digitized image analysis using a BIOCOM image analysis system (Densirag, BioCOM, Les Ulis, France). Grey levels were converted to optical densities by using standard internal curves.
Results
Histology
Bilateral 6-OHDA infusion in the dorsal striatum resulted in a partial loss of [3H]mazindol binding in the anterior dorso-lateral striatum with more complete depletion at posterior levels of the striatum. No loss of [3H]mazindol binding was observed in the nucleus accumbens (Fig. 1A). In one animal, the depletion was asymmetrical and its data were discarded (n = 1 in the 6-OHDA group). For those animals in the implanted groups, only rats showing a placement of the electrode inside the STN (Fig. 1B) were included in the data analysis. After histological verification, six animals were discarded for electrode misplacement (two and four, respectively, from the 6-OHDA-HFS and sham-HFS groups). No tissue damage within the STN was observed.
Fig. 1.
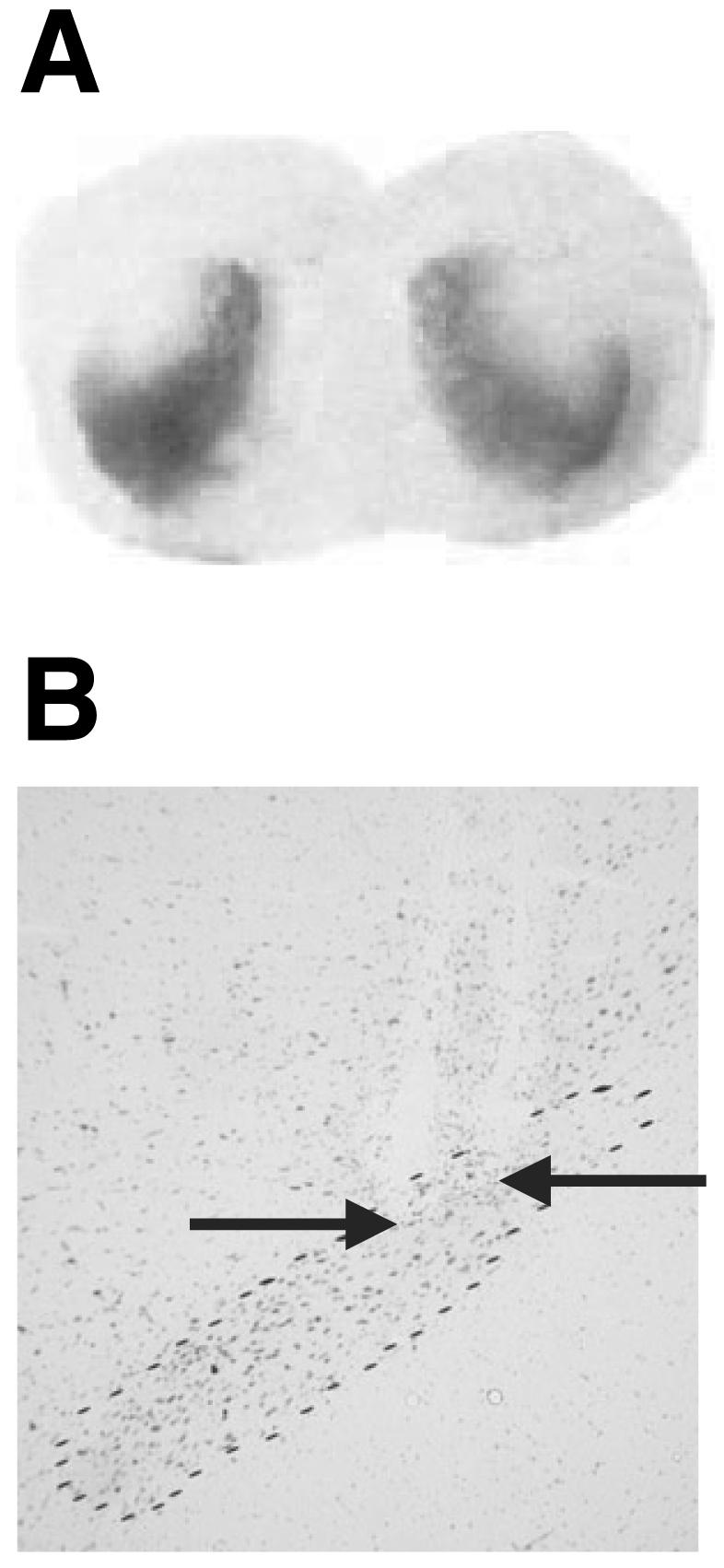
(A) Photomicrographs of a frontal section at the level of the striatum, showing autoradiographic binding for [3H]mazindol in a lesioned animal. (B) Photomicrograph of frontal sections stained with cresyl violet, at the level of the STN outlined by dashed lines, illustrating the location of the tip of the platinium bipolar electrode.
Behavioural results
Preoperative performance
During the preoperative period, performance between groups was homogeneous for most of the variables although performance accuracy was better in the to-be-stimulated groups (77–85% correct responses), omissions (9–13%), correct latency (57–60 cs on average per session), premature responding (12–15 responses per session on average), perseverative responses (28–34 responses per session on average) and perseverative panel pushes (39–83 responses per session on average) [F3,51 = 5.8, P < 0.01 for accuracy; F3,51 = 0.65, 0.71, 0.4, 0.51 and 1.52, P > 0.05 for omissions, latency to make a correct response (i.e. ‘correct latency’), perseverative responses and panel pushes, respectively].
Effects of STN HFS in sham animals
The major effects of STN HFS applied in sham animals were observed during the first block of six sessions during which the stimulation was ON (block ‘stim1’) (Figs 2 and 3). During this first block of STN HFS, accuracy was significantly decreased and the latency to make correct responses was increased as compared with the sham group and preoperative performance (P < 0.01 after significant anova F3,51 = 6.21 and 5.87 and F4,44 = 11.26 and 4.83, respectively, for correct responses and latency). The percentage of omissions was significantly increased in the animals after the implantation of the electrode (block ‘Post’) in comparison with controls and was further increased under stimulation (blocks ‘stim1’ and ‘stim2’) as compared with both controls and preoperative performance (P < 0.05 after significant anova F3,51 = 4.03, 50.93, 5.19 and 5.27 for each block, respectively, and F4,44 = 13.47). No significant effect of STN HFS was observed on premature responding, as it decreased in controls and STN HFS animals for each block following surgery (P < 0.05 and 0.01 after significant block-effect anova F4,52 = 3.43 for sham and F4,44 = 4.22 for sham-HFS). Whereas perseverative responses decreased over time in the control group (P < 0.05 after significant block-effect anova F4,52 = 4.04), they were not significantly increased after STN HFS, leading to a significant difference between sham-HFS and controls for blocks ‘stim2’ and ‘stim OFF’ (P < 0.05 after significant group-effect anova F3,51 = 3.22 and 3.29, respectively). The major effect of STN HFS was an increased number of perseverative panel pushes in the sham-HFS group as compared with controls for blocks ‘stim1’ and ‘stim2’ (P < 0.01 and 0.05 after significant group-effect anova F3,51 = 6.87 and 4.43, respectively) and as compared with the postoperative performance before STN HFS (P < 0.05 after significant block-effect anova F4,44 = 2.67).
Fig. 2.
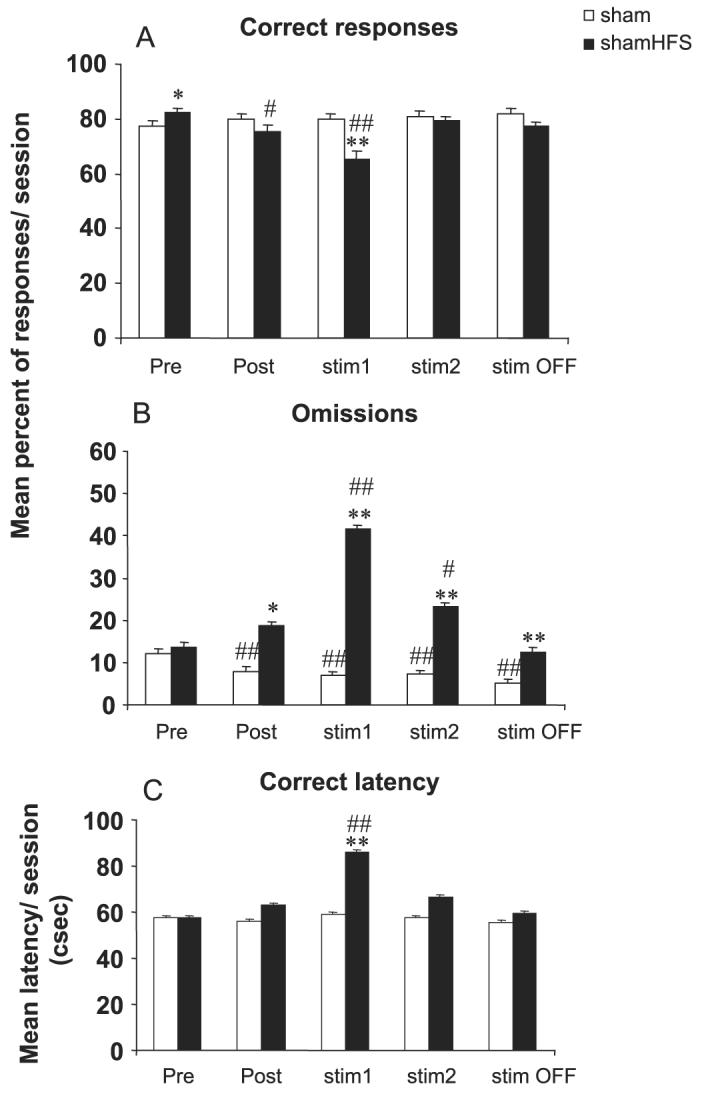
Effects of bilateral STN HFS performed in sham animals on accuracy (A), omissions (B) and latency to make a correct response (C) for sham (n = 14) and sham-HFS (n = 12) animals before surgery (block of six presurgery sessions: Pre), after surgery (block of six postsurgery sessions: Post), under stimulation during two blocks of six daily sessions (stim1 and stim2) and after stimulation (block of six sessions after stimulation was turned off: stim OFF). Vertical bar: SEM. Significant differences compared with sham group, *P < 0.05 and **P < 0.01; compared with preoperative performance, #P < 0.05 and ##P < 0.01.
Fig. 3.
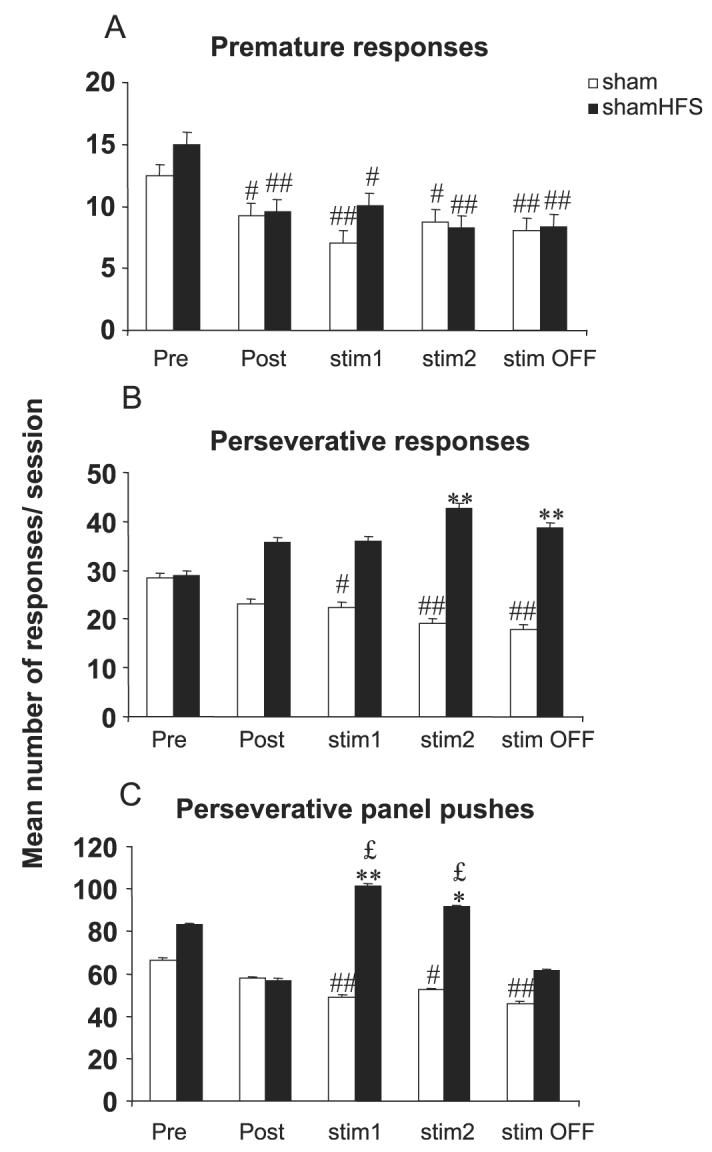
Effects of bilateral STN HFS performed in sham animals on premature (A), perseverative responses (B) and perseverative panel pushes (C) for sham (n = 14) and sham_HFS (n = 12) animals before surgery (block of six presurgery sessions: Pre), after surgery (block of six postsurgery sessions: Post), under stimulation during two blocks of six daily sessions (stim1 and stim2) and after stimulation (block of six sessions after stimulation was turned off: stim OFF). Vertical bar: SEM. Significant differences compared with sham group, *P < 0.05 and **P < 0.01; compared with preoperative performance, #P < 0.05 and ##P < 0.01; compared with postoperative performance, £P < 0.05.
Effects of STN HFS in 6-OHDA lesioned animals
Lesioning the DA terminals into the dorsal striatum induced relatively minor deficits in the task as compared with sham-operated animals or preoperative performance, such as increased omissions (P < 0.05 after anova, F3,51 = 4.03, 50.93, 5.19 and 5.21 for each postoperative block, respectively, and anova F4,60 = 6.00 and anova F4,48 = 18.80 for block effect in the 6-OHDA and 6-OHDA-HFS groups, respectively), increased correct latency (P < 0.05 after anova F3,51 = 2.83 for the postoperative block and anova F4,60 = 6.33 and anova F4,48 = 5.82 for block effect in the 6-OHDA and 6-OHDA-HFS groups, respectively), reduced premature responding (P < 0.05 after anova F4,60 = 5.00 for block effect in the 6-OHDA group), as previously described (Baunez & Robbins, 1999a). The major effects of STN HFS applied in 6-OHDA-lesioned animals were also observed during the first block of six sessions during which the stimulation was ON (block ‘stim1’) (Figs 4 and 5). During this first block of STN HFS, the percentage of omissions was significantly increased as well as the latency to make correct responses as compared with the sham and 6-OHDA groups, preoperative performance (P < 0.05 after significant anova F3,51 = 15.40 and 4.17 and F4,48 = 18.80 and 5.82, respectively, for omissions and latency). No significant effect of STN HFS was observed on premature responding nor on perseverative responses, although there was a significant decrease in premature responding in the 6-OHDA group as compared with preoperative values (P < 0.05 and 0.01 after significant block-effect anova F4,60 = 5.00 for ‘6-OHDA’, while F4,48 = 0.96 P > 0.05 for ‘6-OHDA-HFS’) and a significant increase in perseverative responding as compared with sham-control animals in the ‘6-OHDA’ group at long-term (starting from ‘stim1’) and in the ‘6-OHDA-HFS’ group at block ‘stim2’ only (P < 0.05 after significant group-effect anova F3,51 = .84, 3.22 and 3.29 from blocks ‘stim1’, ‘stim2’ and ‘stim OFF’, respectively).
Fig. 4.
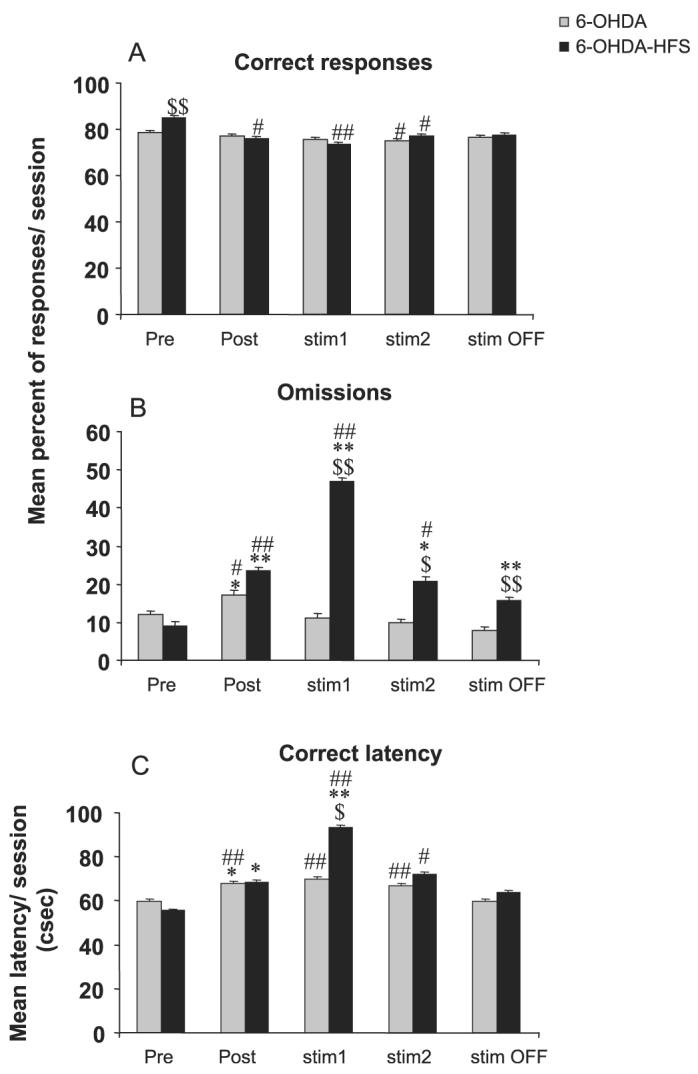
Effects of bilateral STN HFS performed in DA-depleted animals on accuracy (A), omissions (B) and latency to make a correct response (C) for 6-OHDA (n = 16) and 6-OHDA-HFS (n = 13) animals before surgery (block of six presurgery sessions: Pre), after surgery (block of six postsurgery sessions: Post), under stimulation during two blocks of six daily sessions (stim1 and stim2) and after stimulation (block of six sessions after stimulation was turned off: stim OFF). Vertical bar: SEM. Significant differences compared with sham group, *P < 0.05 and **P < 0.01; compared with preoperative performance, #P < 0.05 and ##P < 0.01; compared with 6-OHDA group,$P < 0.05 and $$P < 0.01.
Fig. 5.
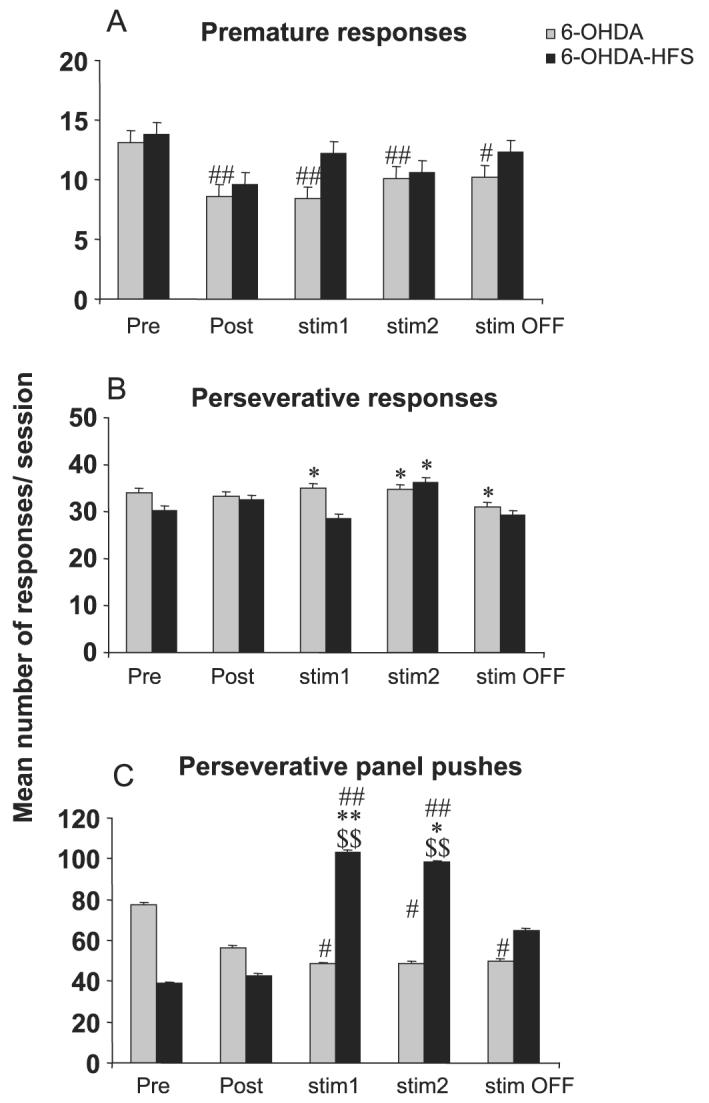
Effects of bilateral STN HFS performed in DA-depleted animals on premature (A), perseverative responses (B), and perseverative panel pushes (C) for 6-OHDA (n = 16) and 6-OHDA-HFS (n = 13) animals before surgery (block of six presurgery sessions: Pre), after surgery (block of six postsurgery sessions: Post), under stimulation during two blocks of six daily sessions (stim1 and stim2) and after stimulation (block of six sessions after stimulation was turned off: stim OFF). Vertical bar: SEM. Significant differences compared with sham group, *P < 0.05 and **P < 0.01; compared with preoperative performance, #P < 0.05 and ##P < 0.01; compared with 6-OHDA group, $P < 0.05 and $$P < 0.01.
The major effect of STN HFS in DA-depleted rats was an increased number of perseverative panel pushes in the ‘6-OHDA-HFS’ group as compared with the ‘sham’ and ‘6-OHDA’ groups for blocks ‘stim 1’ and ‘stim 2’ (P < 0.01 and 0.05 after significant group-effect anova F3,51 = 6.87 and 4.43, respectively) and as compared with the pre- and postoperative performance before STN HFS (P < 0.05 after significant block effect anova F4,48 = 6.15).
Turning the stimulation ‘OFF’
In the sham-HFS group, switching the stimulation off reduced the increased omissions (no longer significantly different as compared with preoperative performance) as well as the perseverative panel pushes (no longer significantly different as compared with sham controls or postoperative performance). However, the long-lasting difference with sham controls on perseverative responses remained (P < 0.01 after group-effect anova F3,51 = 3.29).
In the ‘6-OHDA-HFS’ group, a clear OFF effect was mainly observed on the perseverative panel pushes (no longer significantly different as compared with the sham, 6-OHDA groups or with pre- or postoperative performance).
Discussion
In the present study, we have shown for the first time the effects of HFS of the STN in rats performing a visual attentional task. Our results demonstrate that STN HFS administered to sham-operated controls impairs performance in the task almost as much as do STN lesions (Baunez & Robbins, 1997). Furthermore, STN HFS was found to have a transient detrimental effect in DA-depleted rats, but showed no long-lasting further impairment, although there was an increased perseveration towards the food magazine. Taken together, these observations are in line with the hypothesis that STN HFS mostly mimics STN inactivation.
STN HFS is used in clinics for PD patients. As reported in most reviews, the motor benefits are obvious while cognitive side-effects are less common and difficult to interpret. Indeed, factors other than stimulation can be responsible, such as the selection criteria and operative complications (Parsons et al., 2006). It was thus important to study the effects of STN HFS itself in intact animals in order to understand better the possible cognitive effects of STN stimulation. In this study, we have shown that bilateral STN HFS in intact rats transiently decreased the accuracy of performance, suggesting impaired attention, in line with former results obtained with bilateral STN lesions (Baunez & Robbins, 1997). The percentage of omissions, latency to make correct responses as well as perseverative panel pushes were also increased by STN HFS. All of these effects have also been observed after bilateral STN lesions but were long-lasting whereas most of the effects induced by STN HFS were transient and could have been ameliorated by habituation, suggesting that ‘stim1’ may be more the result of a disturbing effect of the procedure. Significantly in most behavioural situations examined to date, STN lesions (or inactivation via muscimol) have always induced premature responses (Baunez et al., 1995; Baunez & Robbins, 1997, 1999a,b; Phillips & Brown, 1999), but in the present experiment STN HFS did not induce such impulsive behaviour, strongly suggesting that HFS does not simply inactivate the STN. One major difference between STN HFS and STN lesions may be due to the fact that STN HFS was applied acutely during each behavioural session while STN lesions are irreversible and induce permanent changes. In terms of plastic changes consequent to STN lesions, we have shown that STN lesions decrease cortico-striatal activity (Centonze et al., 2005, 2006). Although it has been shown that STN HFS applied chronically for 5 days induces the same effect (Gubellini et al., 2006), it is not known whether this would be the case after acute stimulation as performed in the present study. It is therefore possible that some of the differences observed between acute STN HFS and STN lesions in this behavioural task may account for a difference in reactivity at other levels in the circuit. Given that it has been shown that STN HFS also stimulates GABAergic axon terminals and affects oscillatory activity (Meissner et al., 2005), it is then also possible that STN HFS, via these changes, prevents some of the behavioural effects observed after STN lesions. However, the lack of increase in premature responding may have been obstructed by the fact that these responses decrease over time in the sham groups.
One advantage to apply STN HFS relies on its reversibility, in contrast to a lesion approach. Interestingly, when the stimulation was turned off, some of the behavioural deficits observed were diminished, but some remained persistent. Indeed, the percentage of omissions (for the sham-HFS and 6-OHDA-HHS) as well as the perseverative responses (for the sham-HFS group only) remained different from the sham or the 6-OHDA group after the stimulator was turned off. Although this residual effect of the STN HFS could have been interpreted as a long-lasting effect of the HFS procedure or even as a possible lesion effect, it may well be a non-specific effect as these two variables exhibited a decrease over time in the non-stimulated group. The fact that the increased number of perseverative panel pushes was returned to baseline level during the OFF test rules out the possibility that STN HFS might have induced a lesion at the level of STN, especially as STN lesions (Baunez & Robbins, 1997) or inactivation after intra-STN infusion of muscimol (Baunez & Robbins, 1999b) increase the number of visits to the food magazine. This effect does not seem to depend on an intact DA system, as it is observed in both stimulated groups (sham-HFS and 6-OHDA-HFS). An increased number of visits into the magazine is suggestive of enhanced motivation, as it is significantly reduced by prior feeding (Baunez & Robbins, 1997). Furthermore, we have also shown that STN lesions increase motivation for food reward (Baunez et al., 2002, 2005). In PD patients with STN deep brain stimulation, several cases have been reported to show excessive or impulsive behaviour towards food leading to a weight gain, as well as after STN tumour growth (Barutca et al., 2003; Macia et al., 2004). We have previously shown that STN lesions increase motivation for food, while decreasing motivation for cocaine, suggestive of a dissociation of motivational processes between natural reward vs. drugs of misuse (Baunez et al., 2005). Although incentive–motivational processes are believed to depend on the DA system, we show here that possible motivational effects observed after bilateral STN HFS may be independent of the DA system, the increased perseverations towards the food hopper being evident in both intact and DA-depleted rats.
Although the unilateral DA depletion had induced a severe hemi-neglect in the choice reaction time previously employed (Darbaky et al., 2003), the deficits induced by bilateral striatal depletion were less severe in the present visual attentional task, being related to more motoric slowness (increased omissions and latencies) than to the attentional deficits previously observed (Baunez & Robbins, 1999a). These motor deficits were not alleviated by the bilateral STN HFS, but further impaired during the first block of stimulation, this first block inducing, however, a possible non-specific disturbance as discussed above. In the unilateral model, the hemi-neglect was partially alleviated by unilateral STN HFS in the less severely impaired rats, leading to the conclusion that STN HFS was ineffective at alleviating the cognitive impairments (Darbaky et al., 2003). In a similar task, Temel et al. (2005) have shown that various stimulation parameters were efficient at alleviating motor deficits vs. premature responses considered by the authors as ‘cognitive’ deficits. In the present study, among the deficits induced by bilateral 6-OHDA infusion into the striatum was an increased number of perseverative responses (as compared with the sham-control group). Although STN HFS significantly increased these responses in sham-HFS animals, it was not the case in the 6-OHDA-HFS group. When applied in sham animals STN HFS thus has a disturbing effect that translates into increased perseverative responses, but in animals already affected on this measure by the DA lesion, it has no further negative effect. This can be either the result of a ceiling effect on perseverative responses, but given that these responses even tend to decrease in the 6-OHDA-HFS group for ‘stim1’, it is more likely to be the result of a different mechanism. The perseveration observed after 6-OHDA lesions may be the consequence of the well-known effect of DA depletion to decrease behavioural flexibility, while STN HFS-induced perseveration seems to rely on the integrity of the DA system. The interesting effect here is that it appears that STN HFS did not impair further mild cognitive deficits induced by dopaminergic lesioning, in line with most of the clinical literature (Funkiewiez et al., 2004; Rodriguez-Oroz et al., 2005; Parsons et al., 2006).
In conclusion, this study confirms that STN HFS shares some behavioural effects with STN inactivation by lesions or muscimol infusion, especially by enhancing motivation. However, STN HFS did not impair further mild cognitive deficits resulting from striatal DA depletion.
Acknowledgements
This study was supported by the CNRS, a 5th PCRDT programme funding from the European Community (QLK6- 1999-02173), and by the Association France Parkinson. We thank Drs L. Kerkerian-LeGoff and P. Salin for helpful discussion, and Drs D Eagle and C. Manrique for helping with the experiments and histology, respectively.
Abbreviations
- DA
dopamine
- HFS
high-frequency stimulation
- INI
intertrial interval
- PD
Parkinson's disease
- STN
subthalamic nucleus
- 6-OHDA
6-hydroxydopamine
References
- Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov. Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- Barutca S, Turgut M, Meydan N, Ozsunar Y. Subthalamic nucleus tumor causing hyperphagia – case report. Neurol. Med. Chir. (Tokyo) 2003;43:457–460. doi: 10.2176/nmc.43.457. [DOI] [PubMed] [Google Scholar]
- Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J. Neurosci. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat. Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- Baunez C, Nieoullon A, Amalric M. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J. Neurosci. 1995;15:6531–6541. doi: 10.1523/JNEUROSCI.15-10-06531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur. J. Neurosci. 1997;9:2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999a;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology (Berlin) 1999b;141:57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Koudsie A, Benazzouz A, Piallat B, Krack P, Limousin-Dowsey P, Lebas JF, Pollak P. Deep brain stimulation for Parkinson's disease. Adv. Neurol. 2001;86:405–412. [PubMed] [Google Scholar]
- Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur. J. Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci. Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Rossi S, Picconi B, Pisani A, Bernardi G, Calabresi P, Baunez C. STN lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience. 2005;133:831–840. doi: 10.1016/j.neuroscience.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Gubellini P, De Chiara V, Tscherter A, Prosperetti C, Picconi B, Bernardi G, Calabresi P, Baunez C. Deficits of glutamate transmission in the striatum of experimental hemiballism. Neuroscience. 2006;143:213–221. doi: 10.1016/j.neuroscience.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional disconnection of a prefrontal cortical–dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav. Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Darbaky Y, Forni C, Amalric M, Baunez C. High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur. J. Neurosci. 2003;18:951–956. doi: 10.1046/j.1460-9568.2003.02803.x. [DOI] [PubMed] [Google Scholar]
- Degos B, Deniau JM, Thierry AM, Glowinski J, Pezard L, Maurice N. Neuroleptic-induced catalepsy: electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J. Neurosci. 2005;25:7687–7696. doi: 10.1523/JNEUROSCI.1056-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Temel Y, Visser-Vandewalle V, Blokland A, Hornikx V, Steinbusch HW. Premature responding following bilateral stimulation of the rat subthalamic nucleus is amplitude and frequency dependent. Brain Res. 2004;1008:198–204. doi: 10.1016/j.brainres.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, Chabardes S, Foote K, Benabid AL, Pollak P. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao DM, Benazzouz A, Piallat B, Bressand K, Ilinsky IA, Kultas-Ilinsky K, Benabid AL. High-frequency stimulation of the subthalamic nucleus suppresses experimental resting tremor in the monkey. Neuroscience. 1999;88:201–212. doi: 10.1016/s0306-4522(98)00235-8. [DOI] [PubMed] [Google Scholar]
- Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. Dual effect of high-frequency stimulation on subthalamic neuron activity. J. Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gill SS, Heywood P. Bilateral dorsolateral subthalamotomy for advanced Parkinson's disease. Lancet. 1997;350:1224. doi: 10.1016/s0140-6736(05)63455-1. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Eusebio A, Oueslati A, Melon C, Kerkerian-Le Goff L, Salin P. Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: effects on motor behaviour and striatal glutamate transmission. Eur. J. Neurosci. 2006;24:1802–1814. doi: 10.1111/j.1460-9568.2006.05047.x. [DOI] [PubMed] [Google Scholar]
- Hajji MD, Salin P, Kerkerian-Le Goff L. Chronic dizocilpine maleate (MK-801) treatment suppresses the effects of nigrostriatal dopamine deafferentation on enkephalin but not on substance P expression in the rat striatum. Eur. J. Neurosci. 1996;8:917–926. doi: 10.1111/j.1460-9568.1996.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Annett LE, Ryan LJ, Chiang W, Hidaka S, Torres EM, Dunnett SB. Subthalamic nucleus lesions induce deficits as well as benefits in the hemiparkinsonian rat. Eur. J. Neurosci. 1999;11:2749–2757. doi: 10.1046/j.1460-9568.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Hartlein J, Braver TS, Barch DM, Carl JL, Perlmutter JS. Dopaminergic modulation of response inhibition: an fMRI study. Brain Res. Cogn. Brain Res. 2004;20:438–448. doi: 10.1016/j.cogbrainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Strittmatter SM, Snyder SH. Differential visualization of dopamine and norepinephrine uptake sites in rat brain using [3H]mazindol autoradiography. J. Neurosci. 1985;5:1513–1521. doi: 10.1523/JNEUROSCI.05-06-01513.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Macia F, Perlemoine C, Coman I, Guehl D, Burbaud P, Cuny E, Gin H, Rigalleau V, Tison F. Parkinson's disease patients with bilateral subthalamic deep brain stimulation gain weight. Mov. Disord. 2004;19:206–212. doi: 10.1002/mds.10630. [DOI] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372–2382. doi: 10.1093/brain/awh616. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5:578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed Academic Press; Sydney: 1986. [Google Scholar]
- Phillips JM, Brown VJ. Reaction time performance following unilateral striatal dopamine depletion and lesions of the subthalamic nucleus in the rat. Eur. J. Neurosci. 1999;11:1003–1010. [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berlin) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robledo P, Feger J. Acute monoaminergic depletion in the rat potentiates the excitatory effect of the subthalamic nucleus in the substantia nigra pars reticulata but not in the pallidal complex. J. Neural Transm. Gen. Sect. 1991;86:115–126. doi: 10.1007/BF01250572. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav. Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Salin P, Manrique C, Forni C, Kerkerian-Le Goff L. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J. Neurosci. 2002;22:5137–5148. doi: 10.1523/JNEUROSCI.22-12-05137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellal F, Hirsch E, Lisovoski F, Mutschler V, Collard M, Marescaux C. Contralateral disappearance of parkinsonian signs after subthalamic hematoma. Neurology. 1992;42:255–256. doi: 10.1212/wnl.42.1.255. [DOI] [PubMed] [Google Scholar]
- Temel Y, Visser-Vandewalle V, Aendekerk B, Rutten B, Tan S, Scholtissen B, Schmitz C, Blokland A, Steinbusch HW. Acute and separate modulation of motor and cognitive performance in parkinsonian rats by bilateral stimulation of the subthalamic nucleus. Exp. Neurol. 2005;193:43–52. doi: 10.1016/j.expneurol.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, Savasta M. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur. J. Neurosci. 2000;12:4141–4146. doi: 10.1046/j.1460-9568.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Herzog J, Lorenz D, Volkmann J, Reiff J, Mehdorn M, Deuschl G, Krack P. Differential effects of L-dopa and subthalamic stimulation on depressive symptoms and hedonic tone in Parkinson's disease. J. Neuropsychiatry Clin. Neurosci. 2006;18:397–401. doi: 10.1176/jnp.2006.18.3.397. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch. Neurol. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]


