Abstract
In the last decade, research into the molecular determinants of aging has progressed rapidly and much of this progress can be attributed to studies in invertebrate eukaryotic model organisms. Of these, single-celled yeast is the least complicated and most amenable to genetic and molecular manipulations. Supporting the use of this organism for aging research, increasing evidence has accumulated that a subset of pathways influencing longevity in yeast are conserved in other eukaryotes, including mammals. Here we briefly outline aging in yeast and describe recent findings that continue to keep this “simple” eukaryote at the forefront of aging research.
Introduction
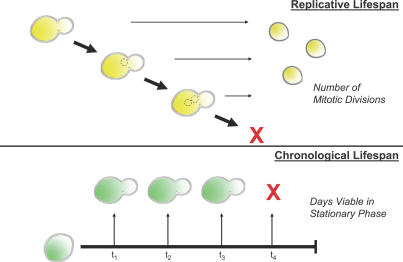
The budding yeast Saccharomyces cerevisiae is a widely used model of cellular and organismal aging [1–4]. The first studies of yeast aging were published over 50 years ago, in which yeast cells were shown to have a finite replicative capacity [5]. Replicative life span was thus defined as the number of daughter cells produced by a mother cell before senescence. A second model of aging has more recently been developed in yeast, termed chronological aging. In contrast to replicative life span (RLS), chronological life span (CLS) is defined as the length of time a yeast cell can survive in a nondividing state [6]. These two models for aging in yeast (Figure 1) provide a unique opportunity to compare and contrast the aging processes of both proliferating and nonproliferating cells in a simple single-celled organism [3].
Figure 1. Schematic for Yeast Replicative and Chronological Aging.
(A) RLS in yeast is measured by the number of mitotic divisions that can arise from a single mother cell. Replicative viability is calculated as the mean number of daughters produced from mothers of a particular strain background before senescence.
(B) CLS is measured by the length of time cells in a stationary culture can remain viable. Viability is calculated by the fraction of the culture able to reenter the cell cycle after an extended state of quiescence.
An interesting parallel has emerged from studies in both yeast aging models linking environmental nutrients to longevity. In the lab, yeast cells are typically grown in media containing high levels of glucose (2%) and abundant amino acids. Independent studies have determined that reducing either the glucose or amino acid concentrations of the media (or both) can increase replicative and chronological life span [7–12]. These different nutrient restriction paradigms have all been referred to as calorie restriction. Calorie restriction is known to increase life span in a variety of organisms other than yeast, including worms, flies, and rodents [13,14]. Given that there is some debate about whether the life-span benefits of these interventions are a direct result of reduced caloric input [15–17], we have chosen to use term dietary restriction (DR) hereafter. There is much interest in determining whether the mechanism(s) by which DR increases longevity in yeast are evolutionarily conserved. A major focus of yeast aging research recently has been directed at understanding the mechanisms that underlie life span extension by DR in yeast.
Dietary Restriction and Sir2: Still Looking for Consensus
Much of the popular interest in yeast aging over the past several years has developed from studies of the silent information regulator 2 (Sir2) family of protein deacetylases (sirtuins). A role for sirtuins in longevity determination was first suggested from work showing that deletion of SIR2 shortens replicative life span [18], while overexpression increases replicative life span [19]. Sir2 orthologs have since been reported to play a similar role in determining the longevity of both worms and flies [20, 21].
In yeast, both overexpression of SIR2 and deletion of FOB1 repress homologous recombination at rDNA repeats. Recombination of rDNA results in the accumulation of extrachromosomal rDNA circles, which can lead to replicative senescence [22]. While it was initially proposed that DR increases RLS in yeast by activating the Sir2 enzyme [11], this model has been challenged by a series of recent studies demonstrating that DR can increase RLS by a SIR2-independent mechanism [23–25]. Although DR fails to increase RLS in a sir2Δ mutant, DR robustly increases the RLS of sir2Δ fob1Δ double mutant cells, demonstrating that Sir2 is not required for life span extension by DR [24]. It remains controversial whether the Sir2 ortholog Hst2 could mediate RLS extension by DR in yeast under specific DR conditions when Sir2 is absent [26,27]; however, recent findings indicate that DR can increase RLS through a mechanism that is independent of all yeast sirtuins [28]. Arguments regarding the relevance of Sir2 in DR have been covered in greater detail in recent reviews and commentaries, and we refer the interested reader to these sources [29–31].
In the chronological aging paradigm Sir2 does not promote longevity and appears to play an antagonistic role in the response to DR [8]. Unlike RLS, deletion of SIR2 does not shorten CLS under normal growth conditions [8]. When cells are subjected to DR, deletion of Sir2 significantly increases CLS [8]. One mechanism that has been proposed for this antilongevity function of Sir2 involves regulating the expression of alcohol dehydrogenase, which is important for metabolism of ethanol late in stationary phase [8]. Whether additional functions of Sir2 are involved as well, such as its role in partitioning of oxidatively damaged proteins between mother and daughter cells [32], remains to be determined.
Apoptosis and Oxidative Stress: Cause or Effect?
An interesting question that has emerged from recent studies is the potential relationship between an apoptosis-like pathway and cellular senescence in yeast. Morphological and molecular features resembling apoptosis in metazoan cells were initially reported in a S. cerevisiae strain with a point mutation in the CDC48 gene [33]. Since this original characterization, apoptotic phenotypes in yeast have been reported to occur under a variety of conditions, including overexpression of pro-apoptotic mammalian BAX [34], by transfer of a stationary culture to a glucose medium lacking additional nutrients to support growth [35], and by treatment with low concentrations of H2O2 [36].
Of particular interest to the field of aging is the effect of oxidative stress on aging cells. Reactive oxygen species accumulate during the diauxic shift and stationary phase, as cells switch from fermentation to oxidative phosphorylation [37]. Reactive oxygen species are also potent stimulators of the mitochondrial cell death pathway, causing loss of mitochondrial membrane potential and export of cytochrome C. Interestingly, either deletion in the yeast ortholog of the apoptosis-inducing factor AIF1 [38] or overexpression of anti-apoptotic mammalian BCL2 [39] rescues the apoptotic phenotype induced by ROS.
With regard to these recent advances, the link between yeast apoptosis and aging remains correlative, but highly intriguing. Both replicatively and chronologically aged cells show markers consistent with apoptotic death [37]. At present, however, it remains unclear whether there exists a causal relationship between apoptosis and senescence in yeast. It has been reported that deletion of the yeast caspase YCA1 results in an increase in chronological longevity, but only after the culture falls below 10% viability [40]. This suggests that apoptosis exerts an effect on longevity only after the majority of cells have already undergone senescence. Thus, for most cells in the population, activation of the apoptosis-like pathway may be a response to the damage leading to senescence. The observation that apoptotic markers are present in both replicative and chronologically senescent cells may be an indication that the ultimate cause of senescence is similar in both dividing and nondividing yeast cells. This would be consistent with the finding that chronologically aged cells have a reduced RLS [41] and that some interventions (e.g., DR or reduced target-of-rapamycin (TOR) signaling) increase both RLS and CLS (see below).
It has also been suggested that apoptosis-like events in chronologically aged cells provide an opportunity for a few individual cells within the population to resume cell division. This argument is based on observations that, at a low frequency, vegetative growth will occasionally be observed late in stationary-phase cultures [42]. It has recently been speculated that this “gasping” effect is an altruistic phenomenon, whereby the majority of cells in an aging population die through a process resembling apoptosis in order to facilitate the outgrowth of a few remaining viable cells [42]. This hypothesis, although controversial, provides an interesting link between replicative and chronological longevity and also suggests a potential mechanism for how an apoptosis-like pathway might evolve in a single-celled eukaryote.
Genome-Wide Screens Elaborate the Importance of Nutrient-Responsive Kinases
An important advance in the field of yeast aging over the last two years has been the development and application of genomic methods for assaying longevity [12,25]. Previously, studies of aging in yeast had been limited to a relatively small number of genes and relied on biased approaches: testing of candidate genes based on prior knowledge or assumptions and screening for secondary phenotypes, such as stress resistance [18,43] or age-associated changes in gene expression that may correlate with longevity [44].
Two reports from genome-wide studies of yeast aging, carried out using a collection of ~4,900 isogenic single-gene deletion strains [45], have identified the nutrient-responsive TOR signaling pathway as an important mediator of both replicative and chronological life span [12,25]. Mutations that decrease TOR activity were found to increase the longevity of both dividing and nondividing yeast cells [12,25]. Interestingly, decreased TOR activity also increases life span in both worms and flies [46–48], suggesting an evolutionarily conserved role for TOR as a conduit linking nutrient status to longevity.
In yeast, TOR acts in concert with other nutrient-responsive kinases, Sch9 and protein kinase A (PKA), to coordinate the cellular response to altered glucose and nitrogen levels [49,50]. Prior studies had implicated roles for both Sch9 and PKA in yeast aging. Similar to inhibition of TOR, deletion of Sch9 increases both replicative and chronological life span [25,43,51]. Likewise, a temperature-sensitive allele of yeast adenylate cyclase (cyr1–1) that decreases PKA activity increases both replicative and chronological life span [9,43]. Suprisingly, deletion of small G proteins that activate the PKA pathway, Ras1 and Ras2, results in opposite effects on the chronological and replicative life spans; deletion of RAS1 increases RLS while slightly decreasing CLS, while deletion of RAS2 decreases RLS, but dramatically extends CLS [9,51,52]. Thus, multiple studies have independently uncovered an important role for these nutrient-responsive signaling pathways in determining yeast longevity.
How might decreased activity of nutrient-responsive kinases lead to increased life span? TOR, Sch9, and PKA play overlapping regulatory roles in several cellular processes that could be of relevance for longevity (Figure 2). In the remainder of this review, we consider which downstream functions are most likely to determine longevity in yeast and, potentially, other organisms.
Figure 2. TOR Kinase Mediates Important Cellular Responses Implicated in Extended Longevity.
During periods of nutrient availability, TOR kinase is activated, leading to G1 progression, translation initiation, increased ribosome biogenesis, and a suppression of autophagy and the stress response.
When TOR is inactivated, either by DR or by the TOR inhibitor rapamycin, a cellular response is initiated that turns down protein translation and cell growth, and increases protein turnover and genes involved in the stress response. The conserved cellular regime that is the result of inactivated TOR kinase increases both replicative and chronological life span in yeast.
Stress response.
One function of TOR, Sch9, and PKA is to repress a general stress response by regulating localization of the transcription factors Msn2 and Msn4 [53–56]. Under conditions of high nutrient availability Msn2/4 are retained in the cytoplasm, where they are unable to activate transcription of starvation-induced stress proteins [53]. Under starvation conditions, or upon treatment with the TOR-inhibitor rapamycin, Msn2/4 relocalize to the nucleus, resulting in enhanced resistance to oxidative and temperature stress. Extension of CLS by the RAS2 deletion appears to be due in part to Msn2/4 activation [9]; however, the chronological life span extension imparted by deletion of Sch9 is independent of Msn2/4; instead, it partly involves activation of RIM15 [43], which mediates entry into stationary phase and activation of stress-responsive genes under those conditions [57].
Although it has not been demonstrated that activation of Msn2/4 is sufficient to either increase replicative or chronological life span, there is indirect evidence supporting the idea that Msn2/4 are involved in chronological life span extension from TOR inhibition. For instance, overexpression of the Msn2/4 target genes SOD1 and SOD2 is sufficient to increase chronological life span [9], suggesting that decreased TOR activity results in increased chronological life span, at least partially, through upregulation of superoxide dismutase activity. Thus, one mechanism by which decreased nutrient availability might slow chronological aging is through an upregulation of stress resistance via activation of Msn2/4 and other pathways.
Interestingly, replicative life span extension from SCH9 deletion, mutations reducing PKA activity, or DR is not dependent on Msn2/4 [11,58]. Thus, given the current available data, the Msn2/4-mediated stress response appears to play an important role in nutrient-mediated chronological, but perhaps not replicative, life-span extension in yeast.
Retrograde response.
The retrograde response has been defined as a mitochondrion-to-nucleus signaling pathway that is activated in response to mitochondrial dysfunction [59,60]. This process is mediated by the transcription factors Rtg1 and Rtg3, which coordinate expression of enzymes involved in anapleurotic production of α-ketoglutarate. The retrograde response has been previously implicated in yeast replicative longevity, with the observation that deletion of mitochondrial DNA (rho0) can increase life span in a retrograde-dependent manner [61]. The relevance of this finding has been difficult to determine, however, because deletion of mitochondrial DNA increases replicative life span in only one out of the six yeast strains in which it has been studied [23,52,61].
In addition to mitochondrial dysfunction, however, retrograde gene expression is also regulated by TOR activity, and treatment of cells with rapamycin induces Rtg1/3-target genes [62,63]. Thus, it is reasonable to speculate that one mechanism by which TOR inhibition could influence replicative longevity is by altering retrograde gene expression. It will be of interest to determine whether Rtg1 and Rtg3 are required for replicative or chronological life span extension from TOR inhibition. Interestingly, deletion of the retrograde target gene IDH2, coding for isocitrate dehydrogenase, also increases yeast replicative life span [25] and two different isocitrate dehydrogenase enzymes are reported to similarly affect longevity in C. elegans [64]. Thus, there is evidence that altering the expression of TOR-regulated retrograde target genes can influence longevity.
Autophagy.
Yet another important function of TOR proteins is to repress autophagy [65,66]. Autophagy is a starvation response in which cellular macromolecules are recycled through vesicular transport and degradation in lysosomal or vacuolar compartments [67]. Autophagy has been implicated in age-associated disease, and autophagy decreases as a function of age in rodents [68–71] More recently, it was shown that increased autophagy is required for full life-span extension in C. elegans in a long-lived daf-2 mutant [72].
Although direct experimental data is lacking, autophagy could be an important mediator of yeast longevity, particularly chronological life span. It is known that yeast cells upregulate autophagy during entry into stationary phase, presumably as an adaptive response to starvation [66]. Consistent with this, several mutants defective for autophagy are short-lived in the chronological aging assay [12]. Treatment of yeast cells with rapamycin or growth under nitrogen starvation induces autophagy [66] and also increases chronological life span [12]. Enhanced autophagy could have several beneficial properties in aging post-mitotic cells, including degradation of oxidatively damaged proteins, inhibition of protein aggregation, and recycling of damaged mitochondria. It will therefore be of interest to determine whether increased autophagy is important for life-span extension from DR or TOR inhibition in either or both of the yeast aging models.
Changes in carbon metabolism.
Yeast cells have evolved to undergo a variety of metabolic changes in response to fluctuating nutrient levels in the environment, many of which are coordinated by TOR, Sch9, and PKA. In particular, yeast respond robustly to decreasing glucose levels by shifting their metabolic state from one that favors fermentation to one that favors respiration. It has been proposed that this shift in carbon metabolism may account for the increase in RLS observed in response to DR [73]. The mechanism postulated by this model was that enhanced respiratory activity would activate Sir2, thus increasing life span. Contrary to this hypothesis, DR increases the RLS of respiratory-deficient cells [23]. This is true, even in cells completely lacking mitochondrial DNA. Similar to the case with SIR2 and DR, there continues to be disagreement about the requirement of respiration for life-span extension by DR, and it has been recently reported that deletion of LAT1, which encodes a component of the mitochondrial pyruvate dehydrogenase complex, also is required for life span extension by DR [74].
There is additional evidence that changes associated with respiratory metabolism can influence both RLS and CLS. For example, overexpression of the glucose-repressible gene HAP4 is sufficient to increase both RLS [73] and CLS [4], even when glucose levels are high. Hap4 is a regulatory subunit required for optimal transcriptional activation by the Hap2/3/5 complex, which induces respiratory genes in response to the available carbon source. Putative Hap2/3/5 binding domains have also been identified in the TSA2 (thiol-specific antioxidant) promoter, which responds to increased oxidative and nitrosative stress [75]. In that report, overexpression of HAP4 was demonstrated to induce TSA2 expression. Thus, in addition to inducing respiration, HAP4 is important for promoting cellular stress resistance. It remains to be determined whether the effects of HAP4 overexpression on replicative and chronological longevity are related to its effects on respiratory metabolism or a different function.
Decreased ribosome biogenesis and translation.
One of the primary functions of TOR, Sch9, and PKA is to modulate protein translation in response to environmental cues [49,76,77]. In yeast, one mechanism by which these kinases regulate translation is by promoting transcription of ribosomal proteins (RPs) and rRNA processing factors. Under conditions of glucose or nitrogen starvation, or upon inhibition of TOR with rapamycin, RP transcription is dramatically reduced and translation in general is impaired [49,63,76,77].
A link between TOR, RPs, and longevity was suggested from the initial results of a genome-wide screen for replicatively long-lived mutants. Replicative life span was determined for 564 single-gene deletion strains randomly chosen from the yeast ORF deletion collection, resulting in the identification of 13 long-lived mutants [25]. In addition to TOR1, the deleted genes from these 13 long-lived strains included two TOR-regulated RP genes, RPL31A and RPL6B . We have since determined that several other RP and rRNA processing factor deletion mutants are also long-lived (our unpublished data), and Chiocetti et al. [78] recently reported that RPS6B and RPL10 similarly regulate replicative longevity. These findings suggest the possibility that one mechanism by which decreased TOR activity can increase replicative life span is by decreasing ribosome function and translation. In this regard, it is noteworthy that mutations in S6 kinase, a downstream target of TOR involved in ribosome maturation, have been reported to increase life span in flies [47], and several recent reports have implicated mRNA translation as a critical determinant of longevity in worms [79–81].
From Yeast to Mammals
It remains an open question how much of the aging process will be conserved from yeast into higher organisms. Clearly, some aspects of aging in yeast are specific to yeast. Others, however, appear to be highly conserved. Life span extension from Sir2-overexpression, TOR-inhibition, Sch9/Akt or DR, for example, has been observed in yeast, worms, and flies. It is likely that several additional conserved longevity factors will be identified from ongoing genome-wide screens in yeast and worms [12,25,52,64,82–85] and studies in mammalian models. If a given gene functions similarly to regulate longevity in yeast, worms, and mice, there is a good chance this function will be conserved in humans. In this way, yeast may serve as a foundation for identifying potential targets for intervening in human longevity and age-associated disease.
The observation that TOR, Sch9/Akt, and PKA could be regulating longevity differently in replicative and chronologically aging yeast cells is noteworthy, given that DR appears to retard a variety of age-associated diseases in tissues of higher animals [14]. The beneficial effects of reduced nutrient signaling may be dependent on the proliferative state of the tissue in question in mammals. Mice subjected to DR are resistant to carcinogenesis and display reduced age-associated pathologies in brain, liver, heart, muscle, and other tissues. How is it that DR has such a broad spectrum of beneficial effects in complex organisms? Based on the studies in yeast described above, we speculate that a few key nutrient-responsive proteins (such as TOR) may serve as evolutionarily conserved gatekeepers to synthesize inputs from the environment into appropriate tissue-specific outputs. For example, in neuronal cells, enhanced degradation of aggregated proteins through increased autophagy might be of particular relevance, whereas in fibroblasts increased resistance to stress or appropriate modulation of ribosome function could be most important. Future studies of the differential responses of different cell types to DR and TOR inhibition will be important for testing this idea.
A growing body of evidence clearly suggests that aging is determined, at least in part, by ancestral evolutionary origins. Due to this conservation, yeast remains a powerful tool for dissecting the genetic and biochemical factors that modulate longevity. As large-scale screens for long-lived yeast deletion mutants draw closer to completion, new and unexpected pathways are being uncovered, bringing a global picture of cellular aging into sharper focus. The knowledge gained from the molecular biology of aging in yeast yields a foundation on which to approach the puzzle of multicellular aging in tissues and in higher organisms.
Acknowledgments
The authors would like to thank members of the Kaeberlein and Kennedy labs for helpful discussions.
Abbreviations
- CLS
chronological life span
- DR
dietary restriction
- PKA
protein kinase A
- RLS
replicative life span
- RP
ribosomal protein
- Sir2
silent information regulator 2
- TOR
target of rapamycin
Footnotes
Matt Kaeberlein is with the Department of Pathology, University of Washington, Seattle, Washington, United States of America. Brian K. Kennedy and Christopher R. Burtner are with the Department of Biochemistry, University of Washington, Seattle, Washington, United States of America.
Funding. Research to identify conserved eukaryotic longevity pathways carried out in the labs of BKK and MK is funded by a grant from The Ellison Medical Foundation. Mechanistic studies of aging in yeast in the lab of BKK is funded by National Institutes of Health Grant R01 AG024287. Biology of aging research in the lab of MK is supported by grants from the American Federation for Aging Research and the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging, NIH P30AG013280.
Competing interests. BKK and MK have a pending patent application for technology for the identification of new aging genes. BKK has a minor stock holding in Elixir Pharmaceuticals.
References
- Bitterman KJ, Medvedik O, Sinclair DA. Longevity regulation in Saccharomyces cerevisiae: Linking metabolism, genome stability, and heterochromatin. Microbiol. and Mol. Biol. Rev. 2003;67:376–399. doi: 10.1128/MMBR.67.3.376-399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski SM. Longevity, genes, and aging: A view provided by a genetic model system. Exp Gerontol. 1999;34:1–6. doi: 10.1016/s0531-5565(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. Longevity and aging in the budding yeast. In: Conn PM, editor. Handbook of models for human aging. Boston: Elvesier Press; 2006. pp. 109–120. [Google Scholar]
- Piper PW. Long-lived yeast as a model for ageing research. Yeast. 2006;23:215–226. doi: 10.1002/yea.1354. [DOI] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae . Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae: Common targets and prevention by calorie restriction. J Biol Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, et al. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Liou LL, Moy VN, Diaspro A, SelverstoneValentine J, et al. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae . Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield (Illinois): Charles C. Thomas; 1988. 436. [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila . Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Mair W, Partridge L. Piper Matthew, Mair William, Linda Partridge on Min K.J, Flatt T, Kulaots I, Tatar M., editors. “Counting calories in Drosophila dietary restriction.”. Exp Gerontol. 2007;2006;42:253–255. doi: 10.1016/j.exger.2007.01.002. Comment by. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Austriaco NR, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae . Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans . Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles-a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, et al. Increased life span due to calorie restriction in respiratory deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:e296. doi: 10.1371/journal.pbio.0020296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers IIIRW, Steffen KK, Westman EA, Hu D, et al. Regulation of yeast replicative life-span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Steffen KK, Hu D, Dang N, Kerr EO, et al. Comment on “HST2 mediates SIR2-independent life-span extension by calorie restriction.”. Science. 2006;312 doi: 10.1126/science.1124608. 1312b. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, et al. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–1864. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Dang N, Kerr EO, Hu D, Steffen KK, et al. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell. 2006;5:505–514. doi: 10.1111/j.1474-9726.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Smith ED, Kaeberlein M. The enigmatic role of Sir2 in aging. Cell. 2005;123:548–550. doi: 10.1016/j.cell.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and SIR2 genes–Towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligr M, Madeo F, Frohlich E, Hilt W, Frohlich KU, et al. Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 1998;438:61–65. doi: 10.1016/s0014-5793(98)01227-7. [DOI] [PubMed] [Google Scholar]
- Granot D, Levine A, Dor-Hefetz E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003;4:7–13. doi: 10.1016/S1567-1356(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, et al. Oxygen stress: A regulator of apoptosis in yeast. J Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Frohlich KU, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissing S, Ludovico P, Herker E, Buttner S, Engelhardt SM, et al. An AIF orthologue regulates apoptosis in yeast. J Cell Biol. 2004;166:969–974. doi: 10.1083/jcb.200404138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J Cell Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Engelhardt S, Herker E, Lehmann N, Maldener C, et al. Apoptosis in yeast: A new model system with applications in cell biology and medicine. Curr. Genet. 2002;41:208–216. doi: 10.1007/s00294-002-0310-2. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Sinclair D, Gordon JI, Guarente L. Passage through stationary phase advances replicative aging in Saccharomyces cerevisiae . Proc Natl Acad Sci U S A. 1999;96:9100–9105. doi: 10.1073/pnas.96.16.9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, et al. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae . J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- D'Mello NP, Childress AM, Franklin DS, Kale SP, Pinswasdi C, et al. Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem. 1994;269:15451–15459. [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, et al. Genetics: Influence of TOR kinase on lifespan in C. elegans . Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Rupes I, Sharom JR, Schneper L, Broach JR, et al. A dynamic transciptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 2004;18:2491–2505. doi: 10.1101/gad.1228804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, et al. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell. 2003;12:1607–1613. doi: 10.1016/s1097-2765(03)00485-4. [DOI] [PubMed] [Google Scholar]
- Sun J, Kale SP, Childress AM, Pinswasdi C, Jazwinski SM. Divergent roles for RAS1 and RAS2 in yeast longevity. J Biol Chem. 1994;269:18638–18645. [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Genes determining yeast replicative life span in a long-lived genetic background. Mech Ageing Dev. 2005;126:491–504. doi: 10.1016/j.mad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD. The Ras and Sch9 pathways regulate stress resistance and longevity. Exp Gerontol. 2003;38:807–811. doi: 10.1016/s0531-5565(03)00113-x. [DOI] [PubMed] [Google Scholar]
- Smith A, Ward MP, Garrett S. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 1998;17:3556–3564. doi: 10.1093/emboj/17.13.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen E, Wanke V, Roosen J, Smets B, Dubouloz F, et al. Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae . Cell Div. 2006;1:3. doi: 10.1186/1747-1028-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae . FEBS Lett. 2004;557:136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Liao X, Butow RA. RTG1 and RTG2: Two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell. 1993;72:61–71. doi: 10.1016/0092-8674(93)90050-z. [DOI] [PubMed] [Google Scholar]
- Kirchman PA, Kim S, Lai CY, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae . Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilova I, Chen CY, Powers T. Mks1 in concert with TOR signaling negatively regulates RTG target gene expression in S. cerevisiae . Curr Biol. 2002;12:389–395. doi: 10.1016/s0960-9822(02)00677-2. [DOI] [PubMed] [Google Scholar]
- Powers T, Dilova I, Chen CY, Wedaman K. Yeast TOR signaling: A mechanism for metabolic regulation. Curr Top Microbiol Immunol. 2004;279:39–51. doi: 10.1007/978-3-642-18930-2_3. [DOI] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, et al. A systematic RNAi screen for longevity genes in C. elegans . Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Sekito T, Ohsumi Y. Autophagy in yeast: A TOR-mediated response to nutrient starvation. Curr Top Microbiol Immunol. 2004;279:73–84. doi: 10.1007/978-3-642-18930-2_5. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- Vittorini S, Paradiso C, Donati A, Cavallini G, Masini M, et al. The age-related accumulation of protein carbonyl in rat liver correlates with the age-related decline in liver proteolytic activities. J Gerontol A Biol Sci Med Sci. 1999;54:B318–B323. doi: 10.1093/gerona/54.8.b318. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: Many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, et al. Autophagy genes are essential for dauer development and life-span extension in C. elegans . Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Easlon E, Tsang F, Dilova I, Wang C, Lu SP, et al. The dihydrolipoamide acetyltransferase is a novel metabolic longevity factor and is required for calorie restriction-mediated life span extension. J Biol Chem. 2007;282:6161–6171. doi: 10.1074/jbc.M607661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett RJ, Bayne AV, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic Biol Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- Martin DE, Soulard A, Hall MN. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell. 2004;119:969–979. doi: 10.1016/j.cell.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae . Mol Biol Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti A, Zhou J, Zhu H, Karl T, Haubenreisser O, et al. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp Gerontol. 2007;42:275–286. doi: 10.1016/j.exger.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans . Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, et al. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans . Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans . Nature. 2007;445:922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:e17. doi: 10.1371/journal.pgen.0010017. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Smith ED, Kennedy BK, Kaeberlein M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech Ageing Dev. 2007;128:106–111. doi: 10.1016/j.mad.2006.11.017. [DOI] [PubMed] [Google Scholar]