Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can selectively kill tumor cells and, in combination with other agents, could enhance tumor therapy. We explored the combined therapeutic effects of a secretable form of (S) TRAIL-induced apoptosis and the downregulation of Bcl-2 in human gliomas. We constructed a lentiviral delivery system: 1) for the expression of short hairpin (sh) RNA to downregulate Bcl-2 and for the expression of S-TRAIL to induce apoptosis in glioma cells; and 2) to follow delivery in vitro and the fate of tumors in real time in vivo. We demonstrate that lentiviral-mediated simultaneous downregulation of Bcl-2 and S-TRAIL-induced apoptosis leads to an increased expression of activated caspase-3 and caspase-7, thus resulting in accelerated S-TRAIL-mediated apoptosis in glioma cells in vitro. Using a highly malignant human glioma model expressing EGFRvIII and firefly luciferase, we show that the combined effect of Bcl-2 downregulation and S-TRAIL-induced apoptosis results in complete eradication of gliomas compared to S-TRAIL monotherapy. These results show that simultaneous triggering of TRAIL-mediated death receptor pathway and downregulation of Bcl-2 by shRNA leads to enhanced eradication of gliomas and serves as a template in developing and monitoring combination therapies for the treatment of drug-resistant cancers.
Keywords: Apoptosis, S-TRAIL, Bcl-2, gliomas, imaging
Introduction
Cancer cells are characterized by self-sufficiency in growth signals, insensitivity to antigrowth signals, sustained angiogenesis, tissue invasion and metastasis, limitless replicative potential, and evasion of apoptosis [1]. The molecular mechanism of drug-induced apoptosis is associated with mitochondrial dysfunction that is characterized by an increase in mitochondrial membrane permeability and is regulated by Bcl-2 [2,3]. The overexpression of Bcl-2 in tumor cells results in antiapoptotic effects triggered through the extrinsic death receptor-dependent pathway [4], but not the death receptor-independent pathway [5]. Targeted repression of Bcl-2 has the potential to facilitate tumor cell apoptosis induced through a number of extrinsic apoptosis-inducing agents, such as tumor necrosis factor-related apoptosisinducing ligand (TRAIL) [6,7], which offers one of the most promising strategies in the field of death ligands and receptors.
Apoptosis initiated by TRAIL involves the binding of TRAIL to death receptors DR4 and DR5, causing the intracellular death domains of these receptors to trimerize [8–10]. Previously, we have engineered a secretable form of TRAIL (S-TRAIL) consisting of an N-terminal fusion of the extracellular domain of Flt3L, a ligand for Flt3 tyrosine kinase receptor, with extracellular domain of TRAIL [9]. We have shown that S-TRAIL has more potent apoptotic effects compared to TRAIL itself, in addition to having a bystander effect for tumor cells both in culture and in mouse models of glioma [10,11]. Recent studies revealed that inhibitors of apoptosis (Bcl-2, XIAP, FLIP, HDAC, or survivin) play an important role in mediating resistance to the apoptotic effects of cytokines TRAIL and TNF-α in melanoma cells [12,13]. Human gliomas overexpress Bcl-2, which has been shown to induce complex changes in glioma cell phenotype and protects glioma cells from various proapoptotic stimuli [14,15].
A vital aspect to successful glioma therapy is to achieve robust and prolonged transgene expression and to follow the fate of tumors overtime. Recently, viral vectors have emerged as an efficient method of integrating transgenes into the host genome. In particular, lentiviral vectors have been used in several studies to genetically modify cells ex vivo as these viruses allow stable integration of transgenes into the host genome irrespective of their state of division [16]. Several approaches to imaging gene delivery and monitoring tumor fate have been described [9,17,18]. Bioluminescent imaging offers high detection sensitivity, low background luminescence from normal tissues, and rapid turnover of luciferase substrates, making this method suited for temporal in vivo imaging of gene expression [19].
In this study, we have engineered S-TRAIL lentiviral vectors and have tested the combined potential of downregulating Bcl-2 by lentiviral-mediated expression of shRNA and by induction of apoptosis by S-TRAIL, released from transduced cells in a highly malignant EGFRvIII glioma model. Furthermore, we have employed noninvasive realtime imaging to follow changes in glioma burden in vivo. To our knowledge, this is the first study to show the effect of downregulating Bcl-2 by lentiviral-mediated shRNA in human gliomas and following the combined effect of S-TRAIL (released within tumors) and Bcl-2 downregulation in real time in vivo. The mode of combined cancer therapy described in this study has three important advantages: 1) the use of glioma cells stably expressing shRNA; 2) the use of an enhanced apoptosis-inducing recombinant S-TRAIL with a bystander effect; and 3) real-time imaging of glioma burden in vivo. This study should immensely help in developing clinically relevant combination therapies in vivo.
Methods
Generation of Lentiviral Vectors
Short hairpin (sh) RNA plasmid constructs for Bcl-2 and green fluorescent protein (GFP) were constructed in pSuper vector (Oligoengine, Seattle, WA) to include the specific 19-bp target sequence for the shBcl-2 construct (5′-TGTGGATGACTGAGTACCTGA-3′) [14] and the shGFP construct (5′-GAACGGCATCAAGGTGAAC-3′) [20] in front of the H1 RNA polymerase III promoter, resulting in pSupershBcl-2 and pSuper-shGFP. H1 promoter shBcl-2 or shGFP fragments were amplified from pSuper vectors using polymerase chain reaction and cloned into CSCRW [derived from CSCGW [21] lentiviral (LV) vector in which GFP sequences were replaced by DsRed2 cDNA sequences], resulting in LV-shBcl-2 and LV-shGFP lentiviral vectors. All constructs were verified by sequencing secretable form of TRAIL (S-TRAIL), which was created by fusing the coding sequences for the extracellular domain of Flt3L, and an isoleucine zipper with the N-terminal of TRAIL [9] was placed under a Cytomegalovirus (CMV) promoter in a vector coexpressing GFP through an internal ribosomal entry sequence (IRES), thus resulting in an LV-S-TRAIL construct. Similarly, cDNA sequences for firefly luciferase (Fluc) were placed under the CMV promoter in a vector coexpressing LacZ through IRES, resulting in the LV-Fluc construct. For a detailed description of lentivirus vector production, see Sena- Esteves et al. [21]. In brief, lentiviral genome (CMVR8.91) was transfected into 293T cells together with an envelopecoding plasmid (VSVG) and vector constructs. LVs were harvested 40 hours posttransfection and concentrated by ultracentrifugation. Titers were determined on 293T cells as transducing units using serial dilutions of vector stocks with 8 µg/ml polybrene (Sigma Chemical, St. Louis, MO).
Generation of shBcl-2-Expressing Glioma Cells
Human glioma lines U87, U-251, Gli36, and Gli36-EGFRvIII (stably expressing the mutant form of EGFR associated with a high malignancy of this tumor type) [19,20] and 293T cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Sigma) at 37°C in a humidified atmosphere with 5% CO2 and 1% penicillin/streptomycin (Invitrogen, Grand Island, NY). Different adherent glioma cell lines were transduced with LV-shBcl-2 or LV-shGFP at multiplicities of infection (MOI) ranging from 1 to 3, and the cells were visualized for DsRed2 expression by fluorescence microscopy. Transduction was carried out for 12 hours at 37°C in the presence of polybrene (8 mg/ml; Sigma). Bcl-2 levels were measured by Western blot analysis 5 days after transduction, as described below. Gli36-EGFRvIII glioma cells were also transduced with LV-Fluc at an MOI of 3; 72 hours later, cells were incubated in a medium containing 150 µg/ml d-luciferin (Biotium, Hayward, CA), and bioluminescence imaging was performed as described below.
S-TRAIL Western Blot Analysis, Enzyme-Linked Immunosorbent Assay (ELISA), and Immunocytochemistry
Human Gli36-EGFRvIII glioma cells and 293T cells were transduced with LV-S-TRAIL vector at an MOI of 1; 36 hours later, a culture medium and the cells were harvested. Proteins were isolated from harvested cells, resolved by sodium dodecyl sulfate-polycrylamide gel electrophoresis (SDSPAGE), and immunoblotted with anti-TRAIL (ProScience, Poway, CA) and anti-cleaved PARP (Cell Signaling, Beverly, MA) antibodies. S-TRAIL concentration in the conditioned culture medium was measured by ELISA with the TRAIL Immunoassay Kit (Biosource International, Camarillo, CA) according to the manufacturer's protocol, using recombinant human TRAIL expressed in Escherichia coli as a standard. Different glioma lines were plated in 96-well plates with five replicate wells and incubated with varying concentrations (0–250 ng/ml) of S-TRAIL obtained from transduced 293T cells. Twenty-four hours later, glioma cell viability was determined as described below. Gli36-EGFRvIII glioma cells were incubated with 80 ng/ml S-TRAIL; 24 hours later, cells were fixed, permeabilized, and incubated with a cleaved caspase-3 antibody (1:100; Cell Signaling) for 1 hour at 37°C. Cells were then washed and incubated with goat anti-rabbit Alexa dye (540 nm)-conjugated secondary antibody (Molecular Probes, Eugene, OR) for 1 hour, washed again, mounted, and examined by fluorescence microscopy.
Cytochrome c and BH3-Interacting Domain Death Agonist (BID) Immunoblotting
For assessing the combined effect of S-TRAIL and Bcl-2 downregulation, an S-TRAIL concentration of 40 ng/ml was used. Gli36-EGFRvIII-shGFP and Gli36-EGFRvIII-shBcl-2 cells were incubated with S-TRAIL (40 ng/ml) for 24 hours, and the total cytochrome c in mitochondrial fractions was analyzed by using cytochrome c assay kit (Calbiochem, San Diego, CA) according to the manufacturer's protocol. Briefly, 5 × 106 of Gli36-EGFRvIII-shGFP and Gli36-EGFRvIII-shBcl-2 cells with or without S-TRAIL treatment was harvested by centrifugation at 700g for 5 minutes. After washes with phosphate-buffered saline (PBS), the cells were resuspended in a 250-µl extraction buffer containing a protease inhibitor mixture and dithiothreitol, and then incubated on ice for 10 minutes, homogenized, and centrifuged at 700g for 10 minutes at 4°C. Supernatant was collected and further centrifuged at 10,000g for 30 minutes at 4°C. Resulting supernatants were harvested and designated as cytosolic fractions, and pellets were resuspended in an appropriate buffer and designated as mitochondrial fractions. Cytochrome c was analyzed using Western blot analysis with cytochrome c monoclonal antibody (Calbiochem) or control antibody against tubulin. Mitochondrial fractions were resolved by SDS-PAGE, transferred to membranes, and immunoblotted with rabbit anti-BID antibody (Cell Signaling, Cambridge, MA).
Caspase-3/7 and Cell Viability Assay
Different glioma lines either expressing shBcl-2 or shGFP were plated at 1 × 104 cells/well in 96-well plates with five replicate wells for each condition. Cells were either left untreated or treated with 40 ng/ml S-TRAIL for 24 hours, and the metabolic activity of the cells was determined using a luminescent adenosine triphosphate (ATP)-based assay (CellTiter GLO; Promega, Madison, WI), according to the manufacturer's instructions. Results were read with a luminometer with a read time of 1 second/well. Glioma cells were also plated as described above, incubated with 40 ng/ml S-TRAIL for 24 hours, and analyzed using ApoONE Homogeneous Caspase 3/7 Assay (Promega), according to the manufacturer's instructions. Samples were read after 1 hour of incubation with the caspase substrate, as described above. S-TRAIL-treated cells were analyzed for viability by luminescent ATP-based assay (CellTiter GLO; Promega) 24 hours later, as described above.
Tumor Models and Glioma Implantations
Athymic nude mice (nu/nu, 6–7 weeks old; Charles River Laboratories, Wilmington, MA) were anesthetized by an intraperitoneal injection of a mix of ketamine (1.6 mg/mice) and xylazine (240 µg/mice) in saline. For correlation studies, different concentrations of Gli36-EGFRvIII-Fl cells (ranging from 5 × 104 to 5 × 106; n = 2 tumors for each cell number) were implanted subcutaneously, and the mice were imaged for Fluc activity 24 hours later, as described below. For assessing the effect of Bcl-2 downregulation and S-TRAIL on glioma proliferation, we employed our previously developed model of assessing the bystander effect of S-TRAIL released within the tumor [10]. Gli36-EGFRvIII-shBcl-2-Fluc or Gli36-EGFRvIII-shGFP-Fluc glioma cells (Gli36-EGFRvIII- Fl cells transduced with either LV-shBcl-2 or LV-shGFP) in midlog phase were harvested by trypsinization, and singlecell suspensions were transduced with LV-S-TRAIL at an MOI of 1 for 6 hours at 37°C. S-TRAIL-transduced cells were washed with PBS, and mice were implanted with: 1) Gli36-EGFRvIII-Fl-shBcl-2; 2) Gli36-EGFRvIII-Fl-shGFP; 3) a mix of 1.5 × 106 of S-TRAIL-transduced cells and 6.0 × 106 of Gli36-EGFRvIII-Fl-shBcl-2 cells; and 4) a mix of 1.5 × 106 of S-TRAIL-transduced cells and 6.0 × 106 of Gli36-EGFRvIII-Fl-shGFP cells, in the two opposite flanks of the mice (n = 10 tumors in each case). Three days after glioma cell implantations, mice were imaged for Fluc activity, as described below. All animal protocols were approved by an institutional review board.
Bioluminescence Imaging in Culture and In Vivo
Fluc-bearing glioma cells were incubated with 150 µg/ml d-luciferin and assayed for luciferase activity using a liquid nitrogen charged cooled device (CCD; Roper Scientific, Trenton, NJ). Mice were given an intraperitoneal injection of D-luciferin (4.5 mg/25 g body weight; prepared in 150 µl of saline) and imaged for Fluc activity for 1 minute after D-luciferin administration using a cryogenically cooled high-efficiency CCD camera system (Roper Scientific). Mice were imaged for Fluc activity on the fourth, sixth, and eighth days after tumor implantation. Postprocessing and visualization were performed as described previously [22].
Tissue Processing and Immunohistochemistry
Two hours following the last luciferase imaging session, mice were sacrificed, tumor tissues were fixed in 10% buffered formalin and embedded in paraffin, and 6-µm sections were cut on a cryostat (CM 3000; Leica Microsystems [Wetzlar, Germany]). The sections were dehydrated in xylene and ethanol then immersed in PBS, and caspase-3 staining was performed according to the manufacturer's protocol using caspase-3 immunostaining kit (Cell Signaling). Immunostained sections were also stained with hematoxylin and eosin.
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed by either Student's t test or analysis of variance (after Bartlett's test of homogeneity of variance), followed by Newman-Keuls correction for multiple comparisons. Differences were considered significant at P < .05.
Results
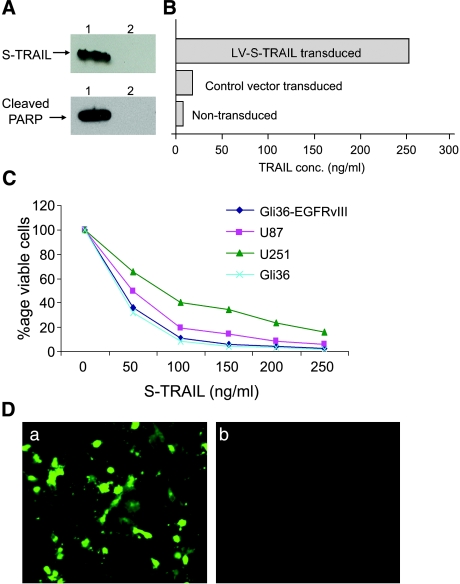
The plasmid constructs used to generate lentivirus vectors (LVs) in this study are diagrammed in Figure 1A. To determine the transduction efficiency of these LVs, 293Tand Gli36 glioma cells were transduced and visualized for DsRed2 and GFP fluorescence. The presence of 90% to 95% DsRed2-positive and GFP-positive glioma cells revealed the high transduction efficiency of LVs (Figure 1B). Gli36-EGFRvIII human glioma cells were transduced with LV-S-TRAIL; 24 hours after transduction, total protein was isolated from tumor cells, fractionated by denaturing SDS-PAGE, and immunoblotted using antiserum against TRAIL. Immuno-reactive proteins corresponding to the size of S-TRAIL were present in the respective LV-S-TRAIL-transduced cells and not in control vector-transduced cells (Figure 2A). Quantitation of TRAIL in the cell supernatant confirmed the secretion of 230 ng/106 cells per 24 hours by glioma cells transduced with LV-S-TRAIL, with no significant amounts produced by nontransduced or control vector-transduced cells (Figure 2B). Cell viability assays on different glioma cell lines treated with varying concentrations of S-TRAIL revealed that both Gli36 and Gli36-EGFRvIII are more susceptible to S-TRAIL-mediated cell killing than are U251 and U87 glioma cells (Figure 2C). Immunocytochemistry and immunoblotting with antibodies detecting cleaved caspase- 3 (Figure 2D) and cleaved PARP (Figure 2A) respectively revealed that lentivirally expressed S-TRAIL induced apoptosis in glioma cells. These results show that TRAIL secreted by lentiviral-transduced cells is active and can induce apoptosis in glioma cells in culture.
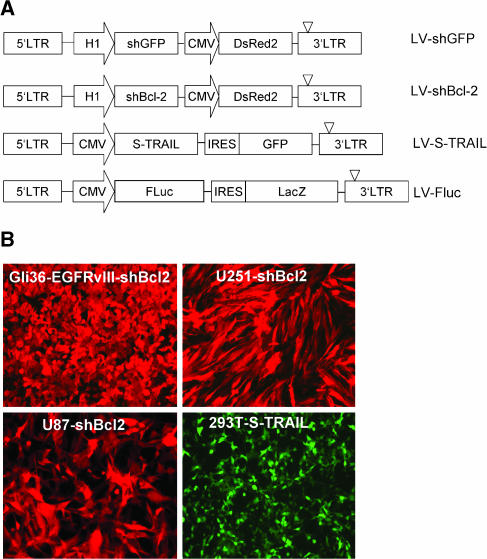
Figure 1.
shRNA, S-TRAIL, and Fluc lentiviral vectors. (A) A self-inactivating lentiviral system based on HIV-1 (CS-CG) [36] was used to construct vectors (i) expressing shRNA against human Bcl-2 and GFP under HI RNA polymerase III promoter and DsRed2 under theCMV promoter; and (ii) expressing S-TRAIL or Fluc under the CMV promoter with an IRES sequence followed by GFP and LacZ, respectively. (B) Human glioma, Gli36-EGFRvIII, U251, and U87 cells transduced in culture with LV-shBcl-2 and 293T cells transduced with LV-S-TRAIL at an MOI of 1 were visualized for DsRed2 or GFP fluorescence. Original magnification, ×10 (B).
Figure 2.
S-TRAIL-induced apoptosis and bystander effect in human glioma cells. (A) Gli36-EGFRvIII glioma cells were transduced with LV-S-TRAIL vector or control vector bearing only IRES-GFP under the CMV promoter; 24 hours after transduction, the cells were harvested, and proteins were resolved by SDS-PAGE and immunoblotted with antibodies against TRAIL and anti-cleaved PARP. Lane 1: S-TRAIL-transduced cell lysates. Lane 2: Control vector-transduced cell lysates. (B) Immunoreactive S-TRAIL protein concentration in the medium of transduced cells, as determined by ELISA. (C) Different glioma lines were plated in 96-well plates with five replicate wells and incubated with varying concentrations (0–250 ng/ml) of S-TRAIL for 24 hours, and glioma cell viability was determined by using a luminescent ATP-based assay. (D) Gli36-EGFRvIII glioma cells were incubated with 80 ng/ml S-TRAIL; 24 hours later, immunohistochemistry was performed with anti-cleaved caspase-3 antibody: (a) S-TRAIL-incubated cells, and (b) control-incubated cells. Original magnification, ×20 (B).
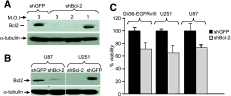
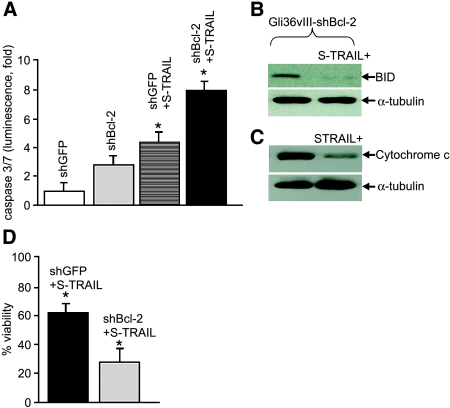
To test whether shBcl-2 could effectively knock down protein levels of Bcl-2 in glioma cells, Gli36-EGFRvIII glioma cells were transduced with LV-shBcl-2 and control LV-shGFP, with MOI ranging from 1 to 3, and the levels of Bcl-2 protein were then determined by immunoblotting after 5 days of transduction using antiserum against Bcl-2. Immunoreactive proteins corresponding to the size of Bcl-2 were present in the respective LV-shGFP-transduced cells and not in LV-shBcl- 2-transduced cells (Figure 3A). The other well-established glioma lines U87 and U251 showed a similar downregulation of Bcl-2 when transduced with shBcl-2 lentiviral vectors (Figure 3B). Viability assays revealed a 25% to 30% reduction in cell viability over a 24-hour period in cells expressing shBcl-2 compared to glioma cells expressing shGFP (Figure 3C). As all the glioma lines tested showed a similar decrease in cell viability, we used Gli36-EGFRvIII glioma cells for further studies, as EGFRvIII expression in gliomas has been previously reported to enhance tumor growth [23]. Next, we investigated whether the combined effect of S-TRAIL and Bcl-2 downregulation would influence the viability of glioma cells in culture. Gli36-EGFRvIII glioma cells were transduced with LV-S-TRAIL with an MOI of 1; 24 hours later, the medium was collected and quantified by ELISA. Both shGFP and shBcl-2-expressing glioma cells were incubated with a medium from LV-S-TRAIL-transduced cells (40 ng /ml) and assessed for caspase-3 activity, cytochrome c release, BID cleavage, and cell viability. At 24 hours, a significantly higher (P < .02) caspase-3/7 activity was assessed in shBcl-2-expressing glioma cells compared to shGFP-expressing cells (Figure 4A). A considerably reduced expression of the 22-kDa BID protein indicating its cleavage was observed in S-TRAIL-treated shBcl-2-expressing cells than in shGFP-expressing glioma cells (Figure 4B). Higher levels of cytochrome c were present in mitochondrial fractions of untreated shBcl-2 glioma cells compared to S-TRAIL-treated shBcl-2 glioma cells (Figure 4C). Furthermore, cell viability in shBcl-2-expressing and shGFP-expressing gliomas treated with S-TRIAL was significantly lower (P < .02) in shBcl-2-expressing cells than in shGFP cells (Figure 4D). These results strongly suggest that downregulation of Bcl-2 sensitizes EGFRvIII-expressing glioma cells to S-TRAIL-mediated apoptosis.
Figure 3.
Downregulation of Bcl-2 using lentiviral-delivered shRNA against Bcl-2. (A) Gli36-EGFRvIII glioma cells were transduced with LV-shBcl-2 or LV-shGFP with different MOI ranging from 1 to 3, and cells were harvested 5 days after transduction. Proteins were resolved by SDS-PAGE and immunoblotted with anti-Bcl-2 and anti-α-tubulin antibodies. (B) U87 and U251 glioma cells were transduced with LV-shBcl-2 or LV-shGFP at an MOI of 3 and harvested after 5 days. Proteins were resolved and blotted as in (A). (C) Different glioma lines were plated; 24 hours later, cell viability was determined using a luminescent ATP-based assay.
Figure 4.
Combined effect of the downregulation of Bcl-2 and S-TRAIL on glioma cells. (A) Gli36-EGFRvIII glioma cells expressing shBcl-2 or shGFP were treated with 40 ng/ml TRAIL; 24 hours later, caspase-3 and caspase-7 activity was determined by using ApoONE Homogeneous Caspase 3/7 Assay. (B) S-TRAIL-treated cells were harvested, and proteins were resolved by SDS-PAGE and immunoblotted with anti-BID antibody. (C) Cytochrome c assays were performed on S-TRAIL-treated cells by isolating mitochondrial fractions resolving the proteins by SDS-PAGE and immunoblotting by anti-cytochrome c antibody. (D) Gli36-EGFRvIII glioma cells expressing shBcl-2 or shGFP were treated with 40 ng/ml TRAIL; 16 hours later, cell viability was determined by using a luminescent ATP-based assay (*P < .02).
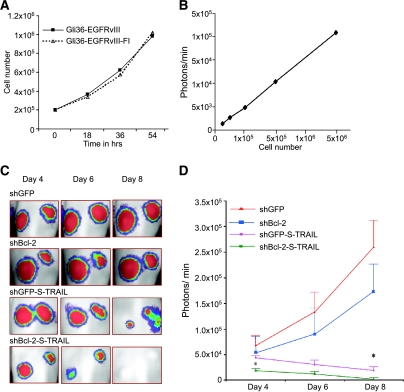
To monitor the therapeutic efficacy of S-TRAIL in vivo, Gli36-EGFRvIII cells were transduced with LV-Fluc, and the resulting Gli36-EGFRvIII-Fl cells were shown to retain the same proliferative capacity as Gli36-EGFRvIII glioma cells (Figure 5A). Different concentrations of Gli36-EGFRvIII-Fl cells (ranging from 5 × 104 to 5 × 106) were implanted subcutaneously, and mice were imaged for Fluc activity 24 hours later. Bioluminescent signal correlated linearly with the number of implanted Gli36-EGFRvIII-Fl cells in the ranges tested (Figure 5B), thus allowing the quantification of cells in vivo in mouse models of glioma. To demonstrate the effect of Bcl-2 downregulation on S-TRAIL-mediated apoptosis in vivo, we also transduced Gli36-EGFRvIII-Fl-expressing shGFP or shBcl-2 glioma cells in culture with LV-S-TRAIL, mixed 20% of LV-S-TRAIL-transduced cells with 80% of Gli36-EGFRvIII-Fl cells, and implanted them subcutaneously into nude mice. The combined effect of Bcl-2 downregulation and S-TRAIL secreted by transduced cells on tumor growth was followed by Fluc bioluminescence imaging on days 4, 6, and 8 after implantation (Figure 5C). A significant decrease in Fluc signal was seen as early as day 4 (P < .02) in shBcl-2-downregulated gliomas; on day 8, the tumors were eradicated in shBcl-2-downregulated gliomas compared to shGFP-downregulated controls treated with S-TRAIL. The results show that downregulation of Bcl-2 and subsequent S-TRAIL treatment result in a substantial decrease in tumor growth compared to implanted tumors with intact Bcl-2 (Figure 5D).
Figure 5.
Enhanced S-TRAIL-induced apoptosis in Bcl-2-downregulated gliomas in vivo. (A) Gli36-EGFRvIII and Gli36-EGFRvIII-Fl cells were grown in culture, and growth over time was compared. (B) Different concentrations of Gli36-EGFRvIII-Fl cells were implanted subcutaneously; 24 hours later, mice were injected intraperitoneally with d-luciferin and imaged for Fluc activity, and the co-relation between the number of implanted cells and photon intensity was plotted. (C) Mice bearing subcutaneous gliomas: (i) Gli36-EGFRvIII-Fl-shGFP, (ii) Gli36-EGFRvIII-Fl-shBcl-2, (iii) Gli36-EGFRvIII-Fl-shGFP-S-TRAIL, and (iv) Gli36-EGFRvIII-FlshBcl-2-S-TRAIL were injected intraperitoneally with d-luciferin and imaged for Fluc activity on days 4, 6, and 8 after implantation. One representative mouse from each group is shown, and each image represents a scan time of 1 minute. A pseudocolor image represents the spatial distribution of photon counts produced by active luciferase within the tumor (D). Fluc bioluminescence intensity of tumors over time, given as the average of tumors in 10 animals, is shown (*P < .02).
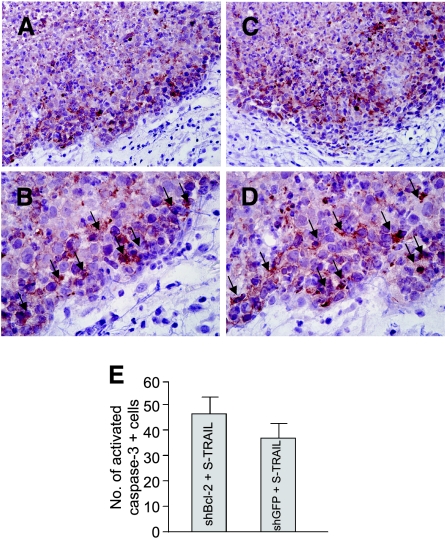
To verify whether S-TRAIL induced the upregulation of caspase-3 in glioma cells, we also performed cleaved caspase-3 staining on shBcl-2 and control tumor sections. A majority of glioma cells in the periphery of the tumor were cleaved caspase-3-positive (Figure 6, A–D), indicating the induction of apoptosis by secreted S-TRAIL from the 20% LV-S-TRAIL-implanted cells. Immunostaining revealed a higher number of cleaved caspase-3-positive cells in Bcl-2-downregulated gliomas than in control gliomas (Figure 6E).
Figure 6.
Immunohistochemistry detects activated caspase-3 in glioma cells. Mice implanted with a mix of LV-S-TRAIL or control vector-transduced and nontransduced Gli36-EGFRvIII-Fl-shGFP or Gli36-EGFRvIII-Fl-shBcl-2 cells (Figure 5) were sacrificed on day 6 after tumor cell implantation, and tumors were sectioned and stained with anti-caspase-3 antibodies. The stained sections were counterstained with hematoxylin. (A and B) Sections from S-TRAIL-expressing Gli36-EGFRvIII-Fl-shGFP gliomas. (C and D) S-TRAIL-expressing Gli36-EGFRvIII-Fl-shBcl-2 gliomas. Caspase-3-stained cells are shown by arrows. (E) The number of activated caspase-3 positive cells was calculated by counting the positive cells in randomly selected field of views under a microscope. Original magnification, ×10 (A and C) and ×40 (B and D).
Discussion
In this study, we have engineered lentiviral vectors with shRNA, apoptosis-inducing agents, and in vivo marker genes, and we have shown that downregulation of antiapoptotic Bcl-2 enhances apoptosis mediated by S-TRAIL in a malignant model of glioma both in vitro and in vivo. Furthermore, we demonstrate that the enhanced apoptosis-inducing ability in Bcl-2-downregulated gliomas can be followed in real time by bioluminescence imaging in live animals.
There is emerging evidence that supports the role of key proteins in controlling cell proliferation, apoptosis, and angiogenesis in the pathogenesis and progression of cancer [24]. Among these proteins, Bcl-2 has been regarded as a potential therapeutic target on the basis of its ability to disrupt apoptosis and to confer resistance to chemotherapy and radiotherapy in cancer cells [12,25–28]. Human glioma cells express a variety of antiapoptotic and proapoptotic Bcl-2 family proteins [29], and overexpression of Bcl-2 has been shown to induce complex changes in glioma cell phenotype in that it not only protects glioma cells from various proapoptotic stimuli [30] but also enhances their motility [31]. We have employed shRNA to inhibit Bcl-2 gene expression as they show features superior to those of antibodies and inhibitors, in part because they are easily applicable to their target, including intracellular factors and even transcription factors. Furthermore, they promise potent gene inhibition with exquisite selectivity, even down to the level of singlenucleotide polymorphisms [32]. Our Bcl-2 immunoblotting results reveal that there is no downregulation of Bcl-2 at an MOI of 1; with higher MOI, this downregulation is specific and significant, and influences the proliferation capacity of the cells. Our findings on the effect of S-TRAIL on Bcl-2- downregulated glioma cells are in line with those of other studies showing the added effect of Bcl-2 downregulation with chemotherapy in vitro and in vivo in other cell types, including non-Hodgkin's lymphoma, Epstein-Barr virus-associated lymphoproliferative disease, malignant melanoma, prostate carcinoma, renal carcinoma, non-small cell lung carcinoma, breast carcinoma, and gastric carcinoma [3].
The use of death ligands for tumor therapy in animal models supports their potential for use in the clinical setting. TRAIL, a type II transmembrane protein, has become an attractive molecule for the treatment of cancers because of its potential to specifically kill tumor cells [8,33]. For efficient tumor therapy, it is important that the tumor cells producing the therapeutic protein not only undergo apoptosis by themselves but also induce apoptosis in surrounding cells. For this purpose, we have engineered a secretable version of TRAIL [9] and have shown that this form of TRAIL (S-TRAIL) has more potent apoptotic effects both in vitro and in vivo [9,10,34]. In this study, we have treated Bcl-2- downregulated gliomas with S-TRAIL and have shown that these gliomas have a significantly enhanced induction of apoptosis both in culture and in vivo. We [10,19,22] and others [35–37] have shown that intratumoral delivery of TRAIL has antitumor activity but requires multiple injections to have an antitumor effect [22]. To circumvent the use of multiple injections and to quantify the subtle changes in tumor growth in S-TRAIL-treated gliomas expressing shBcl-2, we implanted a mix of a defined number of LV-S-TRAIL -transduced glioma cells and nontransduced cells. LV-S-TRAIL-transduced cells produce S-TRAIL within the tumor and induce apoptosis in surrounding tumor cells. The production of S-TRAIL within the tumor allows to quantitate differences between Bcl-2-downregulated and control GFP-downregulated gliomas in vivo. In this study, we have shown that 20% of TRAIL-expressing cells in gliomas is able to significantly reduce tumor burden in Bcl-2-downregulated gliomas in vivo.
The limited availability of noninvasive imaging methods to monitor molecular events has been one of the main limitations in testing the efficacy of various tumor therapy paradigms. In our previous studies, we have shown that we can quantify glioma burden in vivo using noninvasive bioluminescence imaging [22,34]. In the present study, we further demonstrate the sensitivity of luciferase-based bioluminescence imaging and show that we can follow the effect of S-TRAIL on Bcl-2-downregulated gliomas in real time, thus allowing us to quantify the combined effects of Bcl-2 downregulation and the induction of apoptosis by S-TRAIL. Furthermore, the presence of activated caspase-3-positive cells reveals the induction of apoptosis in gliomas through the S-TRAIL-mediated pathway. Most of the glioma is necrotic, except for cells in the tumor border that are still undergoing apoptosis and those cells to which the anti-cleaved caspase-3 antibody binds. The apoptotic process is a dynamic continuum of specific molecular events, and it is difficult to quantitate significant changes in caspase-3 activation between shBcl-2-downregulated and shGFPdownregulated gliomas at a particular time point.
In conclusion, we show that downregulation of Bcl-2 using lentivirally delivered shRNA enhances the apoptosis of glioma cells by S-TRAIL both in vitro and in vivo. The enhanced apoptosis in Bcl-2-downregulated gliomas can be monitored in real time by bioluminescence imaging in live animals. The engineering of clinically approved viral vectors expressing regulatable versions of S-TRAIL and shBcl-2 will be highly useful in tuning up this therapeutic approach.
Footnotes
This work was supported by the American Brain Tumor Association (K.S.), the Goldhirsh Medical Foundation (K.S.), the Catherine and Pappas Award in Neuro-Oncology (K.S.), National Institutes of Health CAP5086355 (R.W.), National Cancer Institute CA86355 (R.W.), and R24 CA92782 (R.W.). N.K. was a fellow of the Deutsche Forschungsgemeinschaft.
Norman Kock and Randa Kasmieh contributed equally to the project.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC, Kroemer G. Mechanisms of mitochondrial membrane permeabilization. Cell Death Differ. 2000;7(12):1145. doi: 10.1038/sj.cdd.4400777. [DOI] [PubMed] [Google Scholar]
- 3.Kim R, Emi M, Tanabe K, Toge T. Therapeutic potential of antisense Bcl 2 as a chemosensitizer for cancer therapy. Cancer. 2004;101(11):2491–2502. doi: 10.1002/cncr.20696. [DOI] [PubMed] [Google Scholar]
- 4.Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103(8):1551–1560. doi: 10.1002/cncr.20947. [DOI] [PubMed] [Google Scholar]
- 5.Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proc Natl Acad Sci USA. 1999;96(26):14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 7.Shah K, Jacobs A, Breakefield XO, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11(15):1175–1187. doi: 10.1038/sj.gt.3302278. [DOI] [PubMed] [Google Scholar]
- 8.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 9.Shah K, Tung CH, Yang K, Weissleder R, Breakefield XO. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64(9):3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- 10.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;111(6):926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Shah K, Tung CH, Chang CH, Slootweg E, O'Loughlin T, Breakefield XO, Weissleder R. In vivo imaging of HIV protease activity in amplicon vector-transduced gliomas. Cancer Res. 2004;64(1):273–278. doi: 10.1158/0008-5472.can-03-1123. [DOI] [PubMed] [Google Scholar]
- 12.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11(8):915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 13.Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia. 2005;7(7):646–657. doi: 10.1593/neo.04655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg. 2001;94(6):978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- 15.Wick W, Wild-Bode C, Frank B, Weller M. BCL-2-induced glioma cell invasiveness depends on furin-like proteases. J Neurochem. 2004;91(6):1275–1283. doi: 10.1111/j.1471-4159.2004.02806.x. [DOI] [PubMed] [Google Scholar]
- 16.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 17.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 18.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9(1):123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 19.Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx. 2005;2(2):215–225. doi: 10.1602/neurorx.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiyar SE, Sun JL, Blair AL, Moskaluk CA, Lu YZ, Ye QN, Yamaguchi Y, Mukherjee A, Ren DM, Handa H, et al. Attenuation of estrogen receptor alpha-mediated transcription through estrogen-stimulated recruitment of a negative elongation factor. Genes Dev. 2004;18(17):2134–2146. doi: 10.1101/gad.1214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122(2):131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Shah K, Tang Y, Breakefield X, Weissleder R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22(44):6865–6872. doi: 10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
- 23.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56(21):5079–5086. [PubMed] [Google Scholar]
- 24.Tortora G, Caputo R, Damiano V, Melisi D, Bianco R, Fontanini G, Veneziani BM, De Placido S, Bianco AR, Ciardiello F. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res. 2003;9(4):1566–1572. [PubMed] [Google Scholar]
- 25.Wacheck V, Losert D, Gunsberg P, Vornlocher HP, Hadwiger P, Geick A, Pehamberger H, Muller M, Jansen B. Small interfering RNA targeting bcl-2 sensitizes malignant melanoma. Oligonucleotides. 2003;13(5):393–400. doi: 10.1089/154545703322617078. [DOI] [PubMed] [Google Scholar]
- 26.Reed JC. Apoptosis mechanisms: implications for cancer drug discovery. Oncology (Williston Park) 2004;18(13 Suppl 10):11–20. [PubMed] [Google Scholar]
- 27.Sellers WR, Fisher DE. Apoptosis and cancer drug targeting. J Clin Invest. 1999;104(12):1655–1661. doi: 10.1172/JCI9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305(5689):1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller M, Rieger J, Grimmel C, Van Meir EG, De Tribolet N, Krajewski S, Reed JC, von Deimling A, Dichgans J. Predicting chemoresistance in human malignant glioma cells: the role of molecular genetic analyses. Int J Cancer. 1998;79(6):640–644. doi: 10.1002/(sici)1097-0215(19981218)79:6<640::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Glaser T, Weller M. Caspase-dependent chemotherapyinduced death of glioma cells requires mitochondrial cytochrome c release. Biochem Biophys Res Commun. 2001;281(2):322–327. doi: 10.1006/bbrc.2001.4349. [DOI] [PubMed] [Google Scholar]
- 31.Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440(3):419–424. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller VM, Xia H, Marrs GL, Gouvion CM, Lee G, Davidson BL, Paulson HL. Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci USA. 2003;100(12):7195–7200. doi: 10.1073/pnas.1231012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11(2):255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 34.Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R, Breakefield XO. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57(1):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 35.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosisinducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165(5):2886–2894. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 36.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, Curley SA, Stephens LC, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61(8):3330–3338. [PubMed] [Google Scholar]
- 37.Seol JY, Park KH, Hwang CI, Park WY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. Adenovirus-TRAIL can overcome TRAIL resistance and induce a bystander effect. Cancer Gene Ther. 2003;10(7):540–548. doi: 10.1038/sj.cgt.7700597. [DOI] [PubMed] [Google Scholar]