Abstract
Effective chemoprevention of lung cancer in high-risk patients through the administration of pharmacologic or nutritional agents is urgently needed. Aerosol inhalation can deliver chemopreventive agents directly to the respiratory tract to inhibit the tumorigenic process. In this study, polyphenon E (PolyE) and (-)-epigallocatechin-3-gallate (EGCG) were administered by aerosol delivery to A/J mice beginning 2 weeks after carcinogen treatment and continuing daily by inhalation throughout the remainder of the study (20 weeks). PolyE decreased tumor load by ∼ 59%. However, EGCG, both at the same dose and at a higher dose, failed to inhibit lung carcinogenesis. These results indicate that aerosol delivery of PolyE, but not EGCG, may be a useful chemopreventive protocol in subjects at high risk for lung cancer.
Keywords: Aerosol, polyphenon E, mouse, lung tumorigenesis, chemoprevention
Introduction
Lung cancer is the leading cause of cancer deaths in both men and women in the United States [1]. Despite improvement in therapy, the cure rate for lung cancer remains low. Chemoprevention offers an important approach to decreasing the incidence of lung cancers. Chemopreventive agents with strong efficacy against lung cancer often cause systemic toxicities and adverse effects by standard delivery modalities. Toxicities can often prevent the clinical use of these agents. Targeting of agents to specific areas within the body can result in better efficacy and lower toxicity. Proper inhalation of agents leads to direct deposition into lung tissues, thereby leading to higher concentrations in the target site and more favorable distribution in comparison to other means of administration (intravenous, oral, or intraperitoneal). Interestingly, aerosol delivery for the chemotherapy of lung cancer in humans has been reported to be effective and to have no adverse effects [2,3]. Aerosol therapies in several human trials showed shrinkage of pulmonary metastases of selected histologic changes from metastatic renal cell cancer [4]. Furthermore, several experiments in animal models used aerosol delivery of chemopreventive agents against lung tumorigenesis [5–10]. For example, aerosol delivery of budesonide at a low dose inhibited all stages of progression from hyperplasia to adenocarcinoma in benzo(a)pyrene [B(a)P]-induced mice lung carcinogenesis without significant systemic toxicity [6,9,10]. Inhaled beclomethasone, at doses starting at 4.8 µg/kg, inhibited lung tumor formation by up to 60% [10]. These results are particularly noteworthy because these chemopreventive agents were given after B(a)P dosing, which is analogous to smokers and former smokers in humans.
Animal studies have shown that green tea is a potent inhibitor of lung tumor development [11–16]. Polyphenon E (PolyE) is a well-defined pharmaceutical-grade mixture of polyphenols that contain at least five different catechins: epicatechin, gallocatechin gallate, epigallocatechin, epicatechin gallate (ECG), and (-)-epigallocatechin-3-gallate (EGCG), with EGCG being the most abundant [17–19]. In this study, animals were exposed to both PolyE and EGCG with a nose-only exposure system. Exposure units are based on the design described by Liao et al. [21]. The aerosol system generated solid particles with uniform size distribution and consistent outputs for both PolyE and EGCG. Aerosol delivery of PolyE, but not EGCG, was found to exhibit significant efficacy against B(a)P-induced mouse lung tumorigenesis.
Materials and Methods
Reagents and Animals
B(a)P (99% pure) and tricaprylin were purchased from Sigma Chemical Co. (St. Louis, MO). B(a)P was prepared immediately before use in animal bioassays by dissolving in tricaprylin. The chemopreventive agents PolyE and EGCG were obtained from Mitsui Norin Co. Ltd. (Shizuoka, Japan). Female A/J mice at 6 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). The use of animals was approved by the Washington University's Institutional Animal Care and Use Committee.
Animal Studies
Female A/J mice were given a single intraperitoneal dose of B(a)P (100 mg/kg body weight, prepared just before injection) in 0.2 ml of tricaprylin. Two weeks after B(a)P injection, the mice were randomized into fours groups: 1) air control group (to account for stress factors during mouse handling procedures in aerosol delivery); 2) vehicle control group; 3) EGCG group (15 mg/ml in water); and 4) PolyE group (15 mg/ml in water). Treatments by aerosol delivery continued for 18 weeks (8 min/day and 5 days/week) (Figure 1A). The mice were exposed singly to aerosol by placing their noses onto the cone of the apparatus. The mice in the air group were placed on aerosol cone for 8 minutes without aerosol treatment to control for potential stress factors affecting tumorigenesis. The body weights of the mice were measured every 3 weeks for the duration of treatments. Mice were sacrificed 20 weeks after exposure to the carcinogen B(a)P by CO2 asphyxiation. Lungs from each mouse were fixed in Tellyesniczky's solution [20] overnight, followed by 70% EtOH. The fixed lungs were evaluated under a dissecting microscope to obtain fixed surface tumor count and individual tumor size. Tumor volume was calculated based on the following formula: mm3 = V = 4/3πr3 [20]. The total tumor volume in each mouse was calculated by the sum of all tumors. Tumor load was determined by averaging the total tumor volume of each mouse in each group.
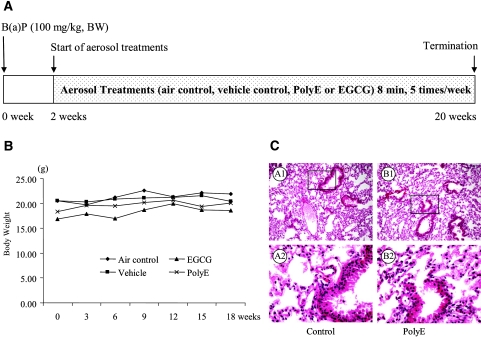
Figure 1.
Protocol and toxicity of aerosolized PolyE and EGCG. (A) Protocol. Two weeks after the intraperitoneal injection of B(a)P, all mice were subjected to aerosol delivery treatment for 8 min/day, 5 days/week. The treatment continued for 18 weeks. (B) Body weight. Mice in all groups were weighed every 3 weeks. No signs of systematic toxicities and adverse effects were observed. (C) Light photomicrographs of lung tissues treated with aerosolized PolyE. Photomicrographs are shown at ×100 and ×400 magnifications on the upper and lower panels, respectively. Black boxes show regions at ×400 magnification. (A1 and A2) Control.
Aerosol Procedure
EGCG or PolyE was aerosolized in a manner similar to that described earlier [21]. Briefly, EGCG or PolyE was dissolved in distilled water and atomized into droplets inside a custom-built glass baffle with an air stream flowing at 0.5 l/min, using a 1.7-MHz ultrasonic driver. The resulting cloud was dried using a reflux dryer [22] and then directed into the nose-only aerosol exposure chamber, which was also custom-built.
Dried aerosol mass output was monitored gravimetrically by collecting aerosol particles with glass microfiber filters (Whatman QMA Grade, 4.7 cm in diameter; Whatman, Florham Park, NJ) in a filter cartridge (FP-050; Schleicher and Schüll, Dassel, Germany) at the exit of a drying column for a fixed period of time. Mass median aerodynamic (MMAD) particle size and geometric standard deviation (GSD) were determined at the exit of the drying column using a cascade impactor (Anderson Mark II Eight-Stage Nonviable Impactor; Graseby-Andersen, Atlanta, GA). Distributions were determined spectrophotometrically, with MMAD particle size and GSD being calculated from a linear regression analysis of a probability plot of cumulative undersize mass versus the logarithm of the cutoff diameter. The stability of catechins was verified by independent measures of output using high-performance liquid chromatography (HPLC).
The mass of inhaled LNP aerosol (Minhaled) was calculated as follows:
where [C]aerosol is the aerosol concentration of catechins, RMV is the respiratory minute volume of the mouse (0.025 l/min, based on Guyton's formula [x]), t is the lengthof timeof aerosol exposure (8 minutes), and W is the mouse weight (0.025 kg). Percent deposition of aerosol within the lung was estimated from assayed tissue mass and inhaled mass, using the following equation:
Following aerosol exposure, the mice were sacrificed by CO2 asphyxiation. Blood was obtained by cardiac puncture, collected into plastic centrifuge tubes, and quench frozen in liquid nitrogen. The lung was severed at the carina and frozen until assayed.
Tissue/Serum Assay Methods
PolyE concentrations in lung and serum samples were determined by HPLC. The HPLC system consisted of an LC-10AD pump, a DGU-14A degasser, an SIL-10A autoinjector affixed with a sample cooler, a CTO-10A column oven, an SPD-10A UV-Vis detector, a C-R5A chromatopac integrator, and an SCL-10A system controller (Shimadzu, Columbia, MD). The HPLC column was SUPELCOSIL LC-18 (25 cm × 4.6 mm, 5 µm), and the wavelength was 277 nm. Stock solutions were prepared by dissolving PolyE and EGCG separately in Vc-EDTA buffer consisting of 20% ascorbic acid and 0.1% EDTA sodium salt in 0.4 M NaH2PO4 buffer at pH 3.6. The mobile phase consisted of 0.1% H3PO4:EtOAc: acetylnitrile (97:2:1). The sample chamber temperature and column temperature were controlled as constants at 4°C and 35°C, respectively. The flow rate of the mobile phase for the first 10 minutes was 1 ml/min, and then 1.5 ml/min from 10 minutes until the end. The peak of EGCG was identified and used to calculate the concentration of PolyE.
Lung tissues were weighed and homogenized in 0.5 ml of ice-cooled Vc-EDTA buffer, and 0.2 ml of homogenate aliquots was transferred into centrifuge tubes containing 10 µl of caffeine (1 mg/ml), followed by vortexing for 30 seconds. A 0.2-ml H2O aliquot, 2 ml of methanol, and 2 ml of acetonitrile were added to the homogenate. Then the sample was vortexed for 2 minutes and centrifuged at 13,000 rpm for 15 minutes at 4°C. Supernatants were transferred and dried under nitrogen gas stream. Residues were reconstituted in 0.5 ml of H2O and centrifuged, and 40 µl of the supernatants was injected into HPLC at 4°C.
An aliquot of 100 µl of each serum sample was transferred into a centrifuge tube containing 20 µl of Vc-EDTA buffer and 10 µl of caffeine (1 mg/ml). After vortexing for 30 seconds, the serum was extracted as above for lung tissue homogenate, except that the residues were reconstituted in 0.2 ml of H2O.
Statistical Analysis
Tumor multiplicity and tumor load were analyzed by two-sided Student's t test using Microsoft Excel 2002 SP-3 (Microsoft, Redmond, WA) to determine differences in the number and in the size of lung tumors per mouse between groups. In all t-tests, the level of statistical significance was set at P < .05.
Results and Discussions
The results of output measurements and aerosol deposition studies are given in Table 1. Inhaled dose represents the total mass that is expected to enter the respiratory tract of mice with a normal breathing pattern, consistent with Guyton's formula for respiratory minute volume. Values depend on aerosol production output, airflow rate, and throughput efficiency in transporting particles to the point of inhalation. Two different inhaled doses of EGCG and PolyE were used. The use of an ultrasonic atomizer provides for a dense aerosol cloud, which largely depends on the properties of the solvent. Thus, no major differences were seen between EGCG and PolyE when solution concentrations were the same. Minor differences may be a result of slight differences in solution viscosity.
Table 1.
Doses and Particle Size Distributions of Aerosolized PolyE and EGCG.
| Aerosol | Inhaled Dose (mg/kg) | MMAD Particle Size (µm) | GSD | Deposited Mass (µg/kg) | Deposition (%) |
| EGCG, 7.5 | 13.4 ± 4.0 | 0.72 | 2.1 | 277 ± 59 | 2.0 |
| EGCG, 15 | 28.0 ± 7.4 | 1.09 | 1.8 | 416 ± 64 | 1.5 |
| PolyE, 7.5 | 16.6 ± 0.9 | 0.92 | 1.8 | 417 ± 64 | 2.5 |
| PolyE, 15 | 29.5 ± 4.8 | 1.28 | 1.7 | 664 ± 82 | 2.3 |
Values represent mean ± SD (n ≥ 3).
Also given in Table 1 are particle size distributions. As can be seen, lower concentrations yielded smaller particles (0.7 and 0.9 µm, in comparison to 1.1 and 1.3 µm), consistent with the fact that the initial drop contained a larger mass and, with drying, would result in a larger dried particle. All things being equal, the diameter should increase with the cube root of the initial mass concentration, which is consistent with the data. GSD is reasonable for ultrasonic atomization and reflects a fairly narrow distribution in production that likely is broadened due to the aggregation of particles in transit. It appears that PolyE yielded somewhat larger mean sizes, which may be due to differences in particle density.
The deposited mass is given in Table 1, which was calculated from the assayed lung concentration of EGCG. For the two doses of EGCG, deposited masses versus body weight were 277 and 417 µg/kg, corresponding to lung concentrations of about 60 and 75 µg/g. In contrast, values for PolyE were higher at 417 and 664 µg/kg. The mass deposited by PolyE consists of about 60% EGCG and 40% other catechins. Consistent with deposited masses and reflecting similar outputs of aerosol device, percent depositions were about 2% and 1.5% for low and high doses of EGCG, and 2.5% and 2.3% for low and high doses of PolyE, respectively. For each compound, a lower percent deposition is consistent with a larger mean particle size; however, a higher deposition was expected for EGCG due to its smaller mean particle size. The concentration of EGCG in the serum was much lower because these animals were sacrificed immediately after exposure, which does not allow for significant transport of EGCG from the lung to the serum. Values ranged from 0.5 µg/ml to a little over 3 µg/ml (less than 1/20 of lung concentrations), reflecting the efficiency of aerosol delivery to the lung.
We did not observe systematic toxicities and other adverse effects within the duration of the bioassays. Two weeks after the injection of B(a)P, the mice were treated by aerosol delivery for 18 weeks. During the experiment, all mice showed great tolerance to treatment with either PolyE or EGCG. No significant difference in body weights or clinical evidence of toxicity was observed (Figure 1B). The aerosol delivery of PolyE and EGCG did not induce noticeable damage to lung tissues (Figure 1C).
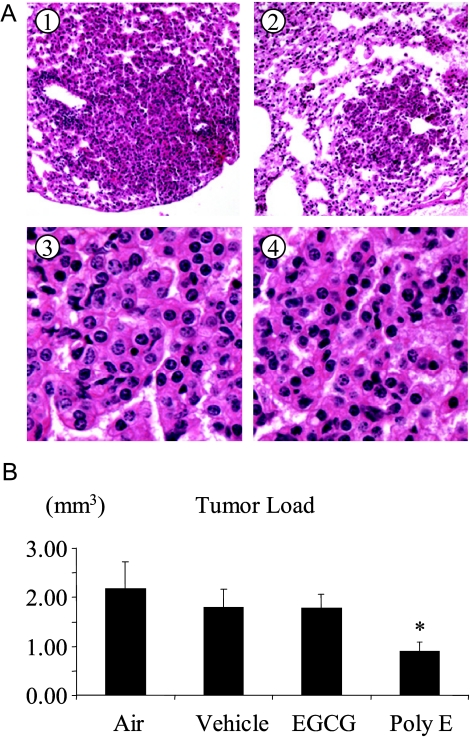
We found that aerosol delivery of PolyE significantly inhibited lung tumorigenesis. EGCG and PolyE (both at the dose of 15 mg/ml in solvent) were used to determine their inhibitory effects on B(a)P-induced lung tumorigenesis. A detailed histopathological examination was conducted to determine the degree of lung tumor progression related to the effect of EGCG and PolyE on tumor development. All lung nodules were diagnosed as lung adenomas (Figure 2A). In the PolyE-treated group, the mice showed a significant decrease in tumor load as 0.9 ± 0.18 mm3 compared to the air and vehicle control groups (2.17 ± 0.55 and 1.78 ± 0.37 mm3, respectively; Figure 2B). Thus, PolyE treatment decreased tumor load by 59% compared with the air control group (P < .05). EGCG treatment did not exhibit significant efficacy. The tumor load in the EGCG group was 1.78 ± 0.28 mm3, similar to those of the vehicle control group and the air group (Figure 2B). As expected in postinitiation protocol, aerosol treatment was initiated 2 weeks after the injection of B(a)P; both EGCG and PolyE groups (6.7 ± 0.7 and 5.91 ± 0.94 tumors/mouse) had tumor multiplicities similar to those of air (6.09 ± 0.64) and vehicle (6.55 ± 0.95) control groups.
Figure 2.
Efficacy of aerosolized PolyE against B(a)P-induced mouse lung tumorigenesis. (A) Lung adenomas. Light photomicrographs of representative adenomas from the control group (A1 and A3) and the PolyE group (A2 and A4) at ×100 and ×400 magnifications, respectively. (B) Effect of PolyE on tumor load. Aerosol delivery of PolyE 2 weeks after B(a)P initiation reduced lung tumor load by 59%. Error bars indicate standard error. *P < .05.
Previous studies have shown that aerosol delivery has the potential advantage of achieving high concentrations of a test agent at the target site with minimum systemic distribution [9]. We have shown here that aerosol delivery of PolyE can be a useful alternative approach for the chemoprevention of lung cancer. Thus, aerosol delivery of PolyE should be considered for further studies in other animal models of lung cancer and in clinical trials.
Green tea has been shown to be chemopreventive in several animal models [11–13,23,24]. However, the effect of the aerosol administration of PolyE and EGCG on lung tumorigenesis has not been determined. In this study, the aerosol delivery of PolyE inhibited tumor load, which is commonly interpreted as tumor growth during tumor progression. With a concentration of 15 mg/ml in water, PolyE decreased tumor load by 59% compared with the control group (Figure 2B). At the same concentration, EGCG did not show any significant effect on tumor load. This was observed despite the fact that EGCG is the main component (about 60%) of PolyE [17–19]. These results indicate that for the chemopreventive efficacy of PolyE from aerosol delivery, EGCG may not be the major contributor for PolyE's inhibitory effect on mouse lung tumorigenesis when given by aerosol delivery. Other components of PolyE, such as epicatechin, gallocatechin gallate, epigallocatechin, and ECG, should be evaluated in the future [14,25,26]. Alternatively, metabolites of EGCG or other catechins, which would not be available to the lung following aerosol administration, may be involved. Furthermore, it is possible that EGCG could still be the active compound, but for its antitumor activity, it may require another component that is present in PolyE formulation and, as such, none of the other components in the PolyE may be biologically active without EGCG or vice versa. Regardless of the nature of the active agent in PolyE, aerosol delivery of PolyE can increase its efficacy by achieving high concentrations of the agent in lung tissues. Tea polyphenols have various biologic activities, including antioxidation, modulation of enzyme systems for metabolizing chemical carcinogens, inhibition of nitrosylation reactions, scavenging of activated metabolites of chemical carcinogens, inhibition of tumor promotion, and induction of apoptosis [15,26,27]. It is likely that some degree of apoptosis and inhibition of cell proliferation may contribute to decreases in tumor load.
Acknowledgement
We thank Yukihiko Hara of Mitsui Norin Co. Ltd. for supplying PolyE and EGCG.
Footnotes
This work was supported, in part, by grants (R01 CA113793 and P01 CA9696401) from the National Cancer Institute, National Institutes of Health.
References
- 1.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 2.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. Br J Cancer. 1993;68:1146–1149. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA, Knight V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 4.Rao RD, Markovic SN, Anderson PM. Aerosol therapy for malignancy involving the lungs. Curr Cancer Drug Targets. 2003;3:239–250. doi: 10.2174/1568009033481895. [DOI] [PubMed] [Google Scholar]
- 5.Kim HW, Park IK, Cho CS, Lee KH, Beck GR, Jr, Colburn NH, Cho MH. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004;64:7971–7976. doi: 10.1158/0008-5472.CAN-04-1231. [DOI] [PubMed] [Google Scholar]
- 6.Estensen RD, Jordan MM, Wiedmann TS, Galbraith AR, Steele VE, Wattenberg LW. Effect of chemopreventive agents on separate stages of progression of benzo[alpha]pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004;25:197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 7.Gautam A, Koshkina N. Paclitaxel (Taxol) and taxoid derivates for lung cancer treatment: potential for aerosol delivery. Curr Cancer Drug Targets. 2003;3:287–296. doi: 10.2174/1568009033481912. [DOI] [PubMed] [Google Scholar]
- 8.Koshkina NV, Waldrep °C, Knight V. Camptothecins and lung cancer: improved delivery systems by aerosol. Curr Cancer Drug Targets. 2003;3:251–264. doi: 10.2174/1568009033481930. [DOI] [PubMed] [Google Scholar]
- 9.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–5492. [PubMed] [Google Scholar]
- 10.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Galbraith AR, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis. 2000;21:179–182. doi: 10.1093/carcin/21.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA, You M. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents Taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]
- 12.Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- 13.Mimoto J, Kiura K, Matsuo K, Yoshino T, Takata I, Ueoka H, Kataoka M, Harada M. (-)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis. 2000;21:915–919. doi: 10.1093/carcin/21.5.915. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Huang MT, Ho CT, Chang R, Ma W, Ferraro T, Reuhl KR, Yang CS, Conney AH. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52:6657–6665. [PubMed] [Google Scholar]
- 15.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamane T, Takahashi T, Kuwata K, Oya K, Inagake M, Kitao Y, Suganuma M, Fujiki H. Inhibition of N-methyl-N′-nitro-N-nitrosoguanidine-induced carcinogenesis by (-)-epigallocatechin gallate in the rat glandular stomach. Cancer Res. 1995;55:2081–2084. [PubMed] [Google Scholar]
- 17.Chang PY, Mirsalis J, Riccio ES, Bakke JP, Lee PS, Shimon J, Phillips S, Fairchild D, Hara Y, Crowell JA. Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon E. Environ Mol Mutagen. 2003;41:43–54. doi: 10.1002/em.10129. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 19.Hirose M, Mizoguchi Y, Yaono M, Tanaka H, Yamaguchi T, Shirai T. Effects of green tea catechins on the progression or late promotion stage of mammary gland carcinogenesis in female Sprague-Dawley rats pretreated with 7,12-dimethylbenz(a)anthracene. Cancer Lett. 1997;112:141–147. doi: 10.1016/s0304-3835(96)04560-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Wang Y, Yao R, Li J, Yan Y, La Regina M, Lemon WL, Grubbs CJ, Lubet RA, You M. Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene. 2004;23:3841–3850. doi: 10.1038/sj.onc.1207496. [DOI] [PubMed] [Google Scholar]
- 21.Liao X, Liang W, Wiedmann T, Wattenberg L, Dahl A. Lung distribution of the chemopreventive agent difluoromethylornithine (DFMO) following oral and inhalation delivery. Exp Lung Res. 2004;30:755–769. doi: 10.1080/01902140490517836. [DOI] [PubMed] [Google Scholar]
- 22.Wiedmann TS, Hitzman CJ. Reflux drying of aerosols. J Aerosol Med. 2004;17:344–353. doi: 10.1089/jam.2004.17.344. [DOI] [PubMed] [Google Scholar]
- 23.Yang CS, Yang GY, Landau JM, Kim S, Liao J. Tea and tea polyphenols inhibit cell hyperproliferation, lung tumorigenesis, and tumor progression. Exp Lung Res. 1998;24:629–639. doi: 10.3109/01902149809087391. [DOI] [PubMed] [Google Scholar]
- 24.Cao J, Xu Y, Chen J, Klaunig JE. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Fundam Appl Toxicol. 1996;29:244–250. doi: 10.1006/faat.1996.0028. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- 26.You M, Bergman G. Preclinical and clinical models of lung cancer chemoprevention. Hematol/Oncol Clin North Am. 1998;12:1037–1053. doi: 10.1016/s0889-8588(05)70040-x. [DOI] [PubMed] [Google Scholar]
- 27.Yang CS, Chung JY, Yang GY, Li C, Meng X, Lee MJ. Mechanisms of inhibition of carcinogenesis by tea. Biofactors. 2000;13:73–79. doi: 10.1002/biof.5520130113. [DOI] [PubMed] [Google Scholar]