Abstract
Infection during the neonatal period commonly induces apnea episodes, and the proinflammatory cytokine IL-1β may serve as a critical mediator between these events. To determine the mechanism by which IL-1β depresses respiration, we examined a prostaglandin E2 (PGE2)-dependent pathway in newborn mice and human neonates. IL-1β and transient anoxia rapidly induced brainstem-specific microsomal prostaglandin E synthase-1 (mPGES-1) activity in neonatal mice. Furthermore, IL-1β reduced respiratory frequency during hyperoxia and depressed hypoxic gasping and autoresuscitation in mPGES-1 wild-type mice, but not in mPGES-1 knockout mice. In wild-type mice, PGE2 induced apnea and irregular breathing patterns in vivo and inhibited brainstem respiratory rhythm generation in vitro. Mice lacking the EP3 receptor (EP3R) for PGE2 exhibited fewer apneas and sustained brainstem respiratory activity, demonstrating that PGE2 exerts its respiratory effects via EP3R. In human neonates, the infectious marker C-reactive protein was correlated with elevated PGE2 in the cerebrospinal fluid, and elevated central PGE2 was associated with an increased apnea frequency. We conclude that IL-1β adversely affects breathing and its control by mPGES-1 activation and PGE2 binding to brainstem EP3 receptors, resulting in increased apnea frequency and hypoxia-induced mortality.
Apnea and sudden infant death syndrome (SIDS) represent major medical concerns in the neonatal population, and infection may play a crucial role in their pathogenesis. Apnea is a common presenting sign of infection in neonates, and mild viral or bacterial infection precedes death in the majority of SIDS victims (1, 2). Proinflammatory cytokines such as IL-1β may serve as key mediators between these events (3). IL-1β is produced during an acute phase immune response to infection and inflammation and evokes a variety of sickness behaviors (for a review, see ref. 4). Previous studies indicate that this immunomodulator also alters respiration and autoresuscitation (5–10). IL-1β induces expression of the immediate-early gene c-fos in respiration-related regions of the brainstem such as the nucleus tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM) (11). However, IL-1β is a large lipophobic protein that does not readily diffuse across the blood–brain barrier (BBB). Furthermore, the NTS and RVLM do not appear to express IL-1 receptor mRNA (12), and IL-1β does not alter brainstem respiration-related neuronal activity in vitro (5). Thus, it is likely that an indirect mechanism underlies the central respiratory effects of IL-1β.
IL-1β binds to IL-1 receptors on vascular endothelial cells of the BBB and induces cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1) activity (for a review, see ref. 13). COX-2 catalyzes the formation of prostaglandin H2 (PGH2) from arachidonic acid, and mPGES-1 subsequently catalyzes the synthesis of prostaglandin E2 (PGE2) from PGH2. PGE2 is then released into the brain parenchyma where it recently has been shown to mediate several central effects of IL-1β, e.g., fever induction (14), behavioral responses (15), and neuroendocrine changes (16). Prostaglandin also appears to mediate the ventilatory effects of IL-1β. We previously showed that indomethacin, a nonspecific COX inhibitor, attenuates the respiratory depression induced by IL-1β (5). PGE2 itself depresses breathing in fetal and newborn sheep in vivo (17–19) and inhibits respiration-related neurons in vitro (5). Furthermore, EP3 receptors (EP3R) for PGE2 are located in the NTS and RVLM (20, 21).
The present study provides evidence that IL-1β adversely affects central respiration via mPGES-1 activation and PGE2 binding to brainstem EP3R, resulting in increased apnea frequency and failure to autoresuscitate after a hypoxic event.
Results
Endogenous Brainstem mPGES-1 Activity and Tonic Respiratory Effect.
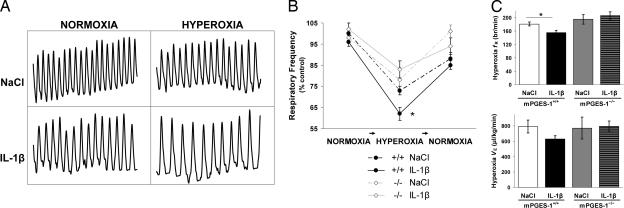
We first examined endogenous PGE2 production and its effects on ventilation in 9-d-old mPGES-1+/+ and mPGES-1−/− mice. Wild-type mice exhibited basal mPGES-1 activity that was higher in the homogenized brainstem than the homogenized cortex (Fig. 1). Breathing during normoxia was similar between genotypes, although respitory frequency (fR) tended to be lower in mPGES-1+/+ mice than mPGES-1−/− mice (Kruskal–Wallis test, P = 0.03; Student's t post hoc test, P = 0.18) [supporting information (SI) Table 2]. The central respiratory drive was examined by a 1-min hyperoxic challenge (100% O2, 1 min). Mice from both genotypes responded to hyperoxia with a reduction in fR (Fig. 2). However, the respiratory depression was greater in mPGES-1+/+ mice than in mPGES-1−/− mice (27 ± 2% vs. 19 ± 3%, respectively).
Fig. 1.
IL-1β and anoxia rapidly induce brainstem mPGES-1. mPGES-1 activity in the microsomal fraction of cortex and brainstem, including endothelial cells of the BBB, was analyzed in 9-d-old mice (n = 33) treated with IL-1β or vehicle and subjected to normoxia or normoxia plus anoxia (100% N2, 5 min). (A) In wild-type mice, mPGES-1 activity was measured at 90 min after NaCl (control) or 90 min and 180 min after IL-1β treatment. Higher endogenous mPGES-1 activity was observed in the brainstem compared with cortex in control mPGES-1+/+ mice. In addition, IL-1β induced mPGES-1 activity in a time-dependent manner. (B) At 90 min, IL-1β-treated mice exhibited approximately 2-fold higher activity in the brainstem compared with saline-treated mice. Anoxia also significantly induced mPGES-1 activity. Moreover, the effects of IL-1β and transient anoxic exposure were additive. When IL-1β-treated mice were exposed to anoxia, 4-fold higher activity was observed in the brainstem compared with control mice. However, mice with genetic deletion of mPGES-1 gene displayed negligible activity in response to IL-1β and anoxia. Data are presented as mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Fig. 2.
IL-1β depresses respiration through mPGES-1 activation. Using whole-body flow plethysmography, basal respiration and the ventilatory response to hyperoxia were examined in 9-d-old mPGES-1 wild-type mice (n = 66) and mPGES-1 knockout mice (n = 34) after i.p. administration of either IL-1β (n = 52) or NaCl (n = 48). (A) Plethysmograph recordings illustrate breathing during normoxia and hyperoxia in wild-type mice given NaCl or IL-1β (5-s period, breath amplitude 1 μl/s). (B and C) All mice responded to hyperoxia with a reduction in fR (breaths per min). IL-1β depressed fR to a greater extent than NaCl in mPGES-1 mice+/+, whereas IL-1β did not alter respiration during normoxia or hyperoxia in mPGES-1−/− mice. mPGES-1+/+ mice exhibited a greater respiratory depression during hyperoxia compared with mPGES-1−/− mice. Data are presented as mean ± SEM. ∗, P < 0.05 compared with mPGES-1+/+ mice given NaCl.
IL-1β and Anoxia Induced mPGES-1 Activity in the Mouse Brainstem.
We also measured the effect of IL-1β and short anoxic exposure (100% N2, 5 min) on mPGES-1 activity in the homogenized brainstem and cortex of 9-d-old mPGES-1+/+, mPGES-1−/−, and EP3R+/+ mice (Fig. 1). IL-1β induced a time-dependent increase in mPGES-1 activity, particularly in the brainstem. Specifically, there was a 2- and 4-fold increase in brainstem mPGES-1 activity at 90 and 180 min, respectively, after IL-1β administration, whereas cortex activity remained unchanged between 90 and 180 min. Anoxic exposure also induced mPGES-1 activity in both brainstem and cortex. Notably, there was an additive effect of IL-β and short anoxic exposure on mPGES-1 activity, which was more pronounced in the brainstem. EP3R wild-type mice displayed similar mPGES-1 activity compared with the mPGES-1 wild-type mice at 90 min after IL-1β. Moreover, the EP3R mice also had higher mPGES-1 activity in the brainstem than the cortex (PGE2: 1,111 ± 49 and 710 ± 44 pmol·min·mg protein, respectively).
IL-1β Depressed Respiration in mPGES-1+/+ Mice, but Not in mPGES-1−/− or EP3R−/− Mice.
To examine the role of PGE2 in mediating the ventilatory effects of IL-1β, we analyzed respiration during normoxia and hyperoxia (100% O2, 1 min) by using flow plethysmography after i.p. administration of IL-1β or vehicle in 9 d-old mPGES-1+/+, mPGES-1−/−, and EP3R−/− mice (Fig. 2 and SI Table 2). All mice, irrespective of treatment, responded to hyperoxic challenge with a reduction in fR, but IL-1β-treated wild-type mice exhibited a greater respiratory depression than vehicle-treated wild-type mice. IL-1β also tended to reduce basal fR in mPGES-1+/+ mice (Kruskal–Wallis test, P = 0.03; Student's t post hoc test, P = 0.17). Conversely, IL-1β did not alter ventilation during normoxia or hyperoxia in mPGES-1−/− or EP3R−/− mice.
IL-1β Worsened Anoxic Survival in Wild-Type Mice, but Not in Mice Lacking mPGES-1 or EP3R.
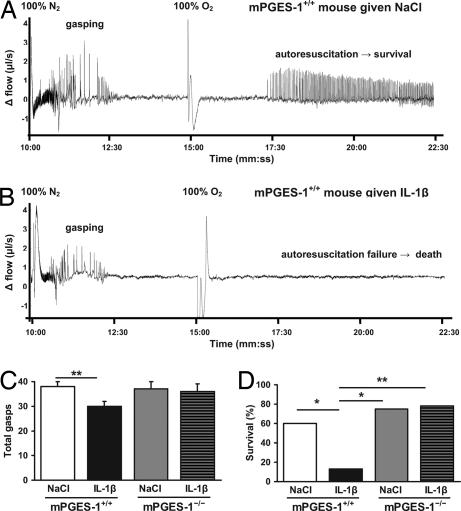
Next, we investigated whether IL-1β affects the hypoxic ventilatory response and autoresuscitation after hypoxic apnea via of a PGE2-mediated mechanism. Using flow plethysmography, respiration during anoxia (100% N2, 5 min) followed by hyperoxia (100% O2, 8 min) was examined beginning at 80 min after i.p. injection of IL-1β or vehicle in mPGES-1+/+, mPGES-1−/−, and EP3R−/− mice (Fig. 3 and SI Table 3). All mice exhibited a biphasic response to anoxia with an initial increase in ventilation (i.e., hyperpnea) followed by a hypoxic ventilatory depression (i.e., primary apnea, gasping, secondary apnea). IL-1β reduced the number of gasps in mPGES-1+/+ mice, but not in mPGES-1−/− mice. IL-1β-treated mPGES-1+/+ mice also tended to have a shorter gasping duration compared with IL-1β-treated mPGES-1−/− mice (Kruskal–Wallis test, P = 0.19; Student's t post hoc test, P = 0.003). Fewer gasps and a shorter gasping duration were correlated with decreased anoxic survival. IL-1β significantly reduced anoxic survival in mPGES-1+/+ mice, but did not decrease survival in mice lacking the mPGES-1 or EP3R genes. IL-1β was unable to affect the hypoxic ventilatory response of EP3R−/− mice.
Fig. 3.
IL-1β reduces anoxic survival via mPGES-1. Nine-day-old mPGES-1+/+ mice (n = 37) and mPGES-1−/− mice (n = 20) were exposed to 5 min of anoxia (100% N2) at 80 min after peripheral administration of IL-1β (n = 29) or vehicle (n = 28). (A) Plethysmograph recording of mPGES-1+/+ mouse given NaCl depicting the initial hyperpnea and subsequent gasping response to anoxia. The mouse autoresuscitated after 100% O2 was administered. (B) Plethysmograph recording of mPGES-1+/+ mouse given IL-1β showing the brief hyperpnea period and subsequent gasping response to anoxia. The mouse failed to autoresuscitate after 100% O2 was administered. (C) The number of gasps tended to differ between groups (Wilcoxon χ2, P = 0.06). When comparing treatment effects within each genotype, IL-1β decreased the number of gasps in wild-type mice, whereas this effect was not observed in mice lacking mPGES-1. (D) IL-1β reduced the survival rate anoxic compared with NaCl in mPGES-1+/+ mice, but not in mPGES-1−/− mice. Data are presented as mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01.
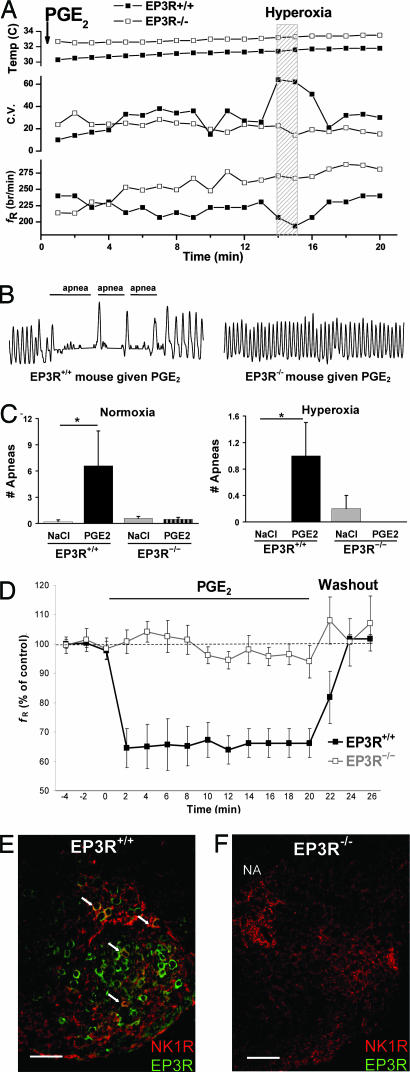
PGE2 Decreased Brainstem Respiration-Related Activity and Induced Apnea via of EP3R.
To better determine whether PGE2 depresses respiration by binding specifically to brainstem EP3 receptors, central respiratory activity was measured using the en bloc brainstem–spinal cord preparation of 2- to 3-d-old EP3R+/+ and EP3R−/− mice after administration of artificial cerebrospinal fluid (aCSF) or PGE2. During control conditions, similar respiratory activity was recorded in preparations from EP3R+/+ and EP3R−/− mice. However, PGE2 reversibly inhibited respiration-related frequency in EP3R+/+ preparations but had no affect on EP3R−/− preparations (Fig. 4). The ability of PGE2 to alter breathing via EP3R was further assessed using flow plethysmography. After intracerebroventricular (i.c.v.) injection of PGE2 or vehicle in EP3R+/+ and EP3R−/− mice, respiration during normoxia and hyperoxia was analyzed (Fig. 4 and Table 1). PGE2 induced a significantly greater apnea frequency and irregular breathing pattern during normoxia and hyperoxia in EP3R+/+ mice, but not in EP3R−/− mice. The mice were subsequently exposed to anoxia followed by hyperoxia, which enabled them to autoresuscitate. All mice continued gasping beyond the 5-min anoxic exposure, and only one of 38 mice failed to autoresuscitate (PGE2-treated EP3R−/− mouse). PGE2 did not alter the gasping response or anoxic survival of EP3R+/+ or EP3R−/− mice compared with vehicle.
Fig. 4.
PGE2 depresses brainstem respiratory activity and induces apnea via brainstem EP3Rs. Respiration was examined in EP3R+/+ (n = 13) and EP3R−/− (n = 25) neonatal mice after administration of PGE2 (n = 19) or NaCl (n = 19). (A) PGE2 was injected i.c.v. at 0 min followed by normoxia and a 1-min hyperoxic challenge in newborn EP3R+/+ (■) and EP3R−/− (□) mice. The EP3R+/+ mouse exhibited a lower fR (breaths per min) and an irregular respiratory rhythm with elevated coefficient of variation (C.V.) during normoxia and hyperoxia due to apneic breathing. In the EP3R−/− mouse, basal fR did not decrease after the postanesthesia period, and there was less variability in the respiratory pattern. No temperature difference or dependency was observed during the first 20 min after i.c.v. administration of PGE2. (B) Plethysmograph recordings (10-s periods with breath amplitude of 1 μl/s) demonstrate apnea episodes in response to PGE2 during normoxia in an EP3R+/+ mouse, but not in an EP3R−/− mouse. (C) In EP3R+/+ mice, PGE2 induced more apneas during normoxia and hyperoxia compared with vehicle. This effect of PGE2 was not observed in EP3R−/− mice. (D) In en bloc brainstem spinal–cord preparations from 2- to 3-d-old EP3R+/+ pups (■, n = 5), PGE2 (20μg/liter) reversibly depressed respiratory rhythm generation to 64 ± 5% of control frequency (fR) (ANOVA repeated measures design, P < 0.01). PGE2 did not affect respiratory activity in preparations from EP3R−/− mice (□, n = 6). (E) In transverse medullary sections, respiration-related neurons within the RVLM ventral to the nucleus ambiguus (NA) and including the preBötC coexpress NK1R (red) and EP3R (green). The arrows indicate EP3R and NK1R colocalization (yellow) in some RVLM respiration-related neurons. (F) NK1R, but no EP3R, expression was identified in an EP3R−/− mouse. (Scale bar, 100 μm.) Data are presented as mean ± SEM. ∗, P < 0.05 compared with EP3R+/+ mice given NaCl.
Table 1.
Respiration during normoxia, hyperoxia, and anoxia in EP3R mice after central PGE2 administration
| Genotype | Treatment | Normoxia |
Hyperoxia |
Hyperpnea |
||||
|---|---|---|---|---|---|---|---|---|
| fR | VT | VE | fR | VT | VE | fR | ||
| EP3R+/+ | NaCl (n = 7) | 281 ± 17 | 3.8 ± 0.4 | 1,065 ± 75 | 234 ± 19 | 7.0 ± 3.0 | 1,598 ± 642 | 327 ± 13 |
| PGE2 (n = 6) | 247 ± 13* | 3.7 ± 0.4 | 901 ± 154 | 190 ± 16 | 4.4 ± 1.1 | 745 ± 102 | 267 ± 11** | |
| EP3R−/− | NaCl (n = 12) | 247 ± 15 | 5.3 ± 0.6 | 1,322 ± 157 | 200 ± 23 | 5.4 ± 0.9 | 1,057 ± 213 | 288 ± 11 |
| PGE2 (n = 13) | 256 ± 10 | 5.2 ± 0.5 | 1,350 ± 129 | 229 ± 9 | 6.7 ± 1.3 | 1,509 ± 299 | 290 ± 9 | |
fR (breaths per min), tidal volume (VT) (μl per breath per g), and minute ventilation (VE)(μl·min·g) during normoxia, hyperoxia (100% O2), and anoxia (100% N2) were examined in 9-d-old EP3R+/+ mice (n = 13) and EP3R−/− mice (n = 25) after i.c.v. injection of PGE2 or vehicle. When comparing treatment effects within each genotype, PGE2 significantly depressed fR during normoxia and hyperpnea in EP3R+/+ mice, but not in EP3R−/− mice. PGE2 also tended to reduce fR during hyperoxia in EP3R+/+ mice (ANOVA, P = 0.11), but not in EP3R−/− mice. Data are presented as mean ± SEM.
*, P < 0.05;
**, P < 0.01.
Finally, we investigated whether respiration-related neurons in the RVLM express EP3R. Specifically, NK1R immunolabeling was used as a tool to identify respiration-related neurons located in the RVLM ventral to the nucleus ambiguous and including the pre-Bötzinger Complex (preBötC) (22–24). We show that these neurons coexpressed NK1R and EP3R (Fig. 4).
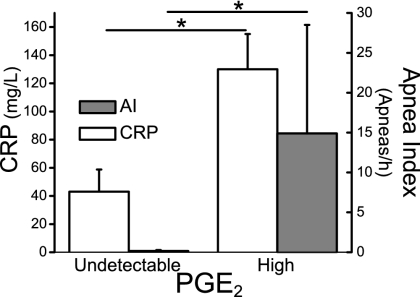
Central PGE2 Concentration Correlated with Increased Apnea Frequency in Human Infants.
To further elucidate the mechanism underlying the association between infection and apnea in human newborns, we examined the association between the infectious marker C-reactive protein (CRP), CSF PGE2 levels, and apnea events in newborn infants. CRP was positively correlated with central PGE2, and there was a positive association between PGE2 concentrations in the CSF and apnea frequency (Fig. 5).
Fig. 5.
PGE2 in CSF is correlated to apnea index in neonates. CSF was collected from infants in the neonatal intensive care unit who had clinical indications for lumbar puncture (n = 12, mean postnatal age 16 ± 4 d; mean gestational age 32 ± 2 week). Infants then underwent a cardiorespiratory recording (duration 9.2 ± 2.4 h). PGE2 concentrations in the CSF were analyzed using a standardized enzyme immunoassay (EIA) protocol and correlated to the infectious marker CRP and apnea index (number of apneas per h). Central PGE2 concentrations were positively correlated to the CRP levels in blood (P = 0.01). Moreover, a striking association was observed between central PGE2 concentrations and apnea index (P < 0.05). Here, we distinguish between undetectable levels of PGE2 (0 ± 0 pg/ml) compared with high levels of PGE2 (52 ± 22 pg/ml). Data are presented as mean ± SEM.
Discussion
In the current investigation, we demonstrate that systemic IL-1β depresses breathing and autoresuscitation via of mPGES-1 activation and PGE2 binding to EP3 receptors in respiration-related regions of the brainstem (Fig. 6). Additionally, severe hypoxia rapidly induces mPGES-1 activity, indicating that endogenous PGE2 may modulate brainstem respiratory neurons during hypoxia in the newborn period. Lastly, we reveal a correlation between infection, central PGE2, and apnea events in human neonates.
Fig. 6.
Model for IL-1β-induced respiratory depression and autoresuscitation failure via a PGE2-mediated pathway. During a systemic immune response, the proinflammatory cytokine IL-1β is released into the peripheral blood stream. It binds to its receptor (IL-1R) located on endothelial cells of the BBB. Activation of IL-1R induces the synthesis of PGH2 from arachidonic acid (AA) via COX-2 and the synthesis of PGE2 from PGH2 via of the rate-limiting enzyme mPGES-1. PGE2 is released into the brain parenchyma and binds to its EP3R located in respiratory control regions of the brainstem, e.g., NTS and the RVLM. This results in depression of central respiration-related neurons and breathing, which may fatally decrease the ability to gasp and autoresuscitate during hypoxic events.
PGE2 Exerts a Tonic Effect on Brainstem Respiratory-Related Activity via mPGES-1.
Our study clearly demonstrates an endogenous expression of mPGES-1 activity, particularly in the brainstem. mPGES-1 is expressed mainly by endothelial cells along the BBB (25). A constitutive and rapidly inducible expression of mPGES-1 at endothelial cells overlying the brainstem, near crucial respiration-related centers, suggests an important role of PGE2 in control of breathing. The significant respiratory depression in wild-type mice compared with mice lacking mPGES-1 during hyperoxia also provides evidence that endogenous PGE2 has a tonic effect on respiratory rhythmogenesis during the perinatal period. This finding is consistent with evidence that prostaglandin synthesis inhibitors, which block endogenous prostaglandin production, increase fetal breathing movements and central respiration during early postnatal life (26–28). Developmental changes occur in the modulatory effects of prostaglandin with an initial inhibition of ventilation during the perinatal period (18, 26, 27, 29) followed by smaller changes in respiration with increasing age (19). However, PGE2 may still disrupt regular breathing with induction of apnea at older ages (19). Developmental changes could be secondary to alterations in brainstem PGE2 receptor expression beyond the perinatal period, although EP3R gene and protein are expressed in adult rodent RVLM (20, 21, 30). In addition, even though prostaglandin binding density may decrease, it is located in the same brainstem regions at all ages (31). Further investigation of the ontogenesis of EP3R expression and mechanisms underlying potential developmental changes in the respiratory effects of PGE2 (e.g., posttranslational EP3R modification, suprapontine influences) is warranted but outside the scope of the present study.
Transient Anoxia Induces mPGES-1 Activity in the Mouse Brain.
PGE2 also appears to play a crucial role in the respiratory response to anoxia. A short anoxic exposure increased mPGES-1 activity in the homogenized mouse brain. This rapid increase in mPGES-1 activity in vivo is a new finding but is consistent with evidence that anoxia induces PGE2 production in mice cortical sections ex vivo and prostaglandin H synthase-2 mRNA expression in the piglet brain (32, 33). Transient asphyxia similarly increases PGE2 concentrations in the newborn guinea pig brain, and this effect is inhibited by pretreatment with indomethacin (34).
No known mechanisms of mPGES-1 enzyme regulation may explain the rapid changes in mPGES-1 activity revealed here. Induced gene expression is unlikely to occur during such a short anoxic event. However, posttranscriptional regulation of constitutively expressed mPGES-1, e.g., phosphorylation, is a potential etiology. Stabilization of mPGES-1 mRNA is another possibility, as previously shown with COX-2 mRNA in a human cell system (35) and recently in cardiac myocytes (36). Further investigation is required to clarify the underlying mechanism.
IL-1β Alters Respiration via mPGES-1 Activation and PGE2 Binding to Brainstem EP3 Receptors.
We reveal that mPGES-1 activation is necessary for IL-1β to depress central respiration. First, IL-1β increased brainstem mPGES-1 activity in a time-dependent manner. Second, IL-1β depressed respiration in mPGES-1+/+ mice, but not in mPGES-1−/− mice. Indomethacin, by blocking prostaglandin synthesis, has been shown to similarly attenuate the effects of IL-1β on basal respiration (5). In addition, we provide evidence that after mPGES-1 activation, newly synthesized PGE2 exerts the respiratory actions of IL-1β centrally. We show here that PGE2 hindered breathing in wild-type mice, consistent with studies demonstrating that PGE2 depresses respiration in fetal and newborn animals (18, 29, 37). Moreover, these effects occur centrally because PGE2 did not alter peripheral chemosensitivity in vivo and directly inhibited brainstem respiratory activity in vitro. These findings are in accordance with data showing that PGE2 inhibits respiration-related neurons in neonatal rats (5) and similarly inhibits fetal breathing movements in sheep after sham-operation or denervation of the carotid sinus and vagus nerve (38).
Furthermore, the modulatory effects of PGE2 occur via binding to brainstem EP3 receptors. IL-1β was unable to alter respiration in EP3R−/− mice. PGE2 induced apnea and irregular breathing in vivo in EP3R+/+ mice, but not in EP3R−/− mice. Finally, the presence of EP3 receptors was required to inhibit brainstem respiration-related rhythmic activity in vitro. Although the specific prostaglandin receptor subtype EP3R has been localized to the NTS and RVLM (20, 21), no prior studies have shown that the respiratory effects of prostaglandin occur via action at these receptors and that they are expressed in respiration-related neurons.
IL-1β Inhibits Autorescuscitation and Anoxic Survival via mPGES-1 and EP3R.
This study demonstrates that PGE2 also plays a crucial role in mediating the anoxic ventilatory effects of IL-1β. IL-1β inhibited autoresuscitation after hypoxic apnea in wild-type mice, but not in mice lacking mPGES-1 or EP3R. These findings are consistent with our previous investigation showing that indomethacin attenuates the adverse effects of IL-1β on hypoxic gasping and anoxic survival in neonatal rats (5).
Our data suggests that PGE2 induced by IL-1β as well as hypoxia selectively modulates respiration-related neurons in the RVLM, including the preBötC, via EP3R. Other neuromodulators, including PGE1, have been shown to inhibit preBötC neurons and slow respiration-related rhythm (22, 23), and preBötC lesions may disrupt anoxic gasping and evoke central apneas and ataxic breathing (39, 40). Moreover, these respiration-related neurons were recently shown to be critical for adequate response to hypoxia, maintaining brainstem homeostasis with gasping and autoresuscitation and thus restoring oxygen levels (41). PGE2-induced depression of this vital brainstem neuronal network, e.g., during an infectious response, could result in gasping and autoresuscitation failure and ultimately death.
Correlation Between Infectious Parameters, PGE2, and Apnea in Human Neonates.
Apnea is a common presenting sign of sepsis in the neonatal population (1), yet the mechanism underlying this association remains unclear. Here, we show that the infectious marker CRP is correlated with elevated PGE2 levels in the CSF of human neonates. Importantly, we also demonstrate that PGE2 is associated with an increased apnea frequency. These findings suggest that infection depresses respiration in human neonates through systemic release of cytokines followed by the biosynthesis and central action of PGE2. The mechanism described here could explain previous reports showing an independent association between CRP levels and the apnea/hypopnea index in children with sleep apnea (42) as well as a positive correlation between IL-1β concentrations in pharyngeal secretions of human infants and clinical severity of apnea (8). Transient apneas are also a common side effect of prostaglandin treatment in human neonates (43), which may be due to activation of EP3 receptors in brainstem respiration-related centers. Furthermore, our data provide an explanation for the positive correlation between central apneas and urine PGE metabolites in newborn infants (44).
Inflammatory mediators have been proposed as important markers for detecting infection and asphyxia in newborns. The rapid synthesis of PGE2 in response to cytokine and hypoxic stimulation may make it particularly useful in the diagnosis and surveillance of infants with increased apneas due to suspected infection or asphyxia. Studies to evaluate the potential diagnostic benefits of monitoring PGE2 compared with other infectious markers such as CRP are necessary.
The present discoveries have important treatment implications for neonatal apnea related to infection because the adverse effects of IL-1β were attenuated by selectively deleting the mPGES-1 and EP3R genes. Indomethacin has been used previously to treat apnea of prematurity (45). However, indomethacin causes multiple adverse effects in the newborn population (46), and thus treatment modalities selectively targeting mPGES-1 or EP3 receptors could be more beneficial.
Future investigations examining the mechanism described in the present study may improve our understanding of the epidemiological and scientific evidence linking infection, apnea, autoresuscitation failure, and sudden infant death syndrome (SIDS).
Materials and Methods
Subjects.
Neonatal mice of the inbred DBA/1lacJ strain (n = 158) (Jackson Laboratory, Bar Harbor, ME) and C57BL/6 strain (n = 75) were used. The mPGES-1 and EP3R genes were selectively deleted in knockout mice as described in refs. 47 and 48. Infants (mean gestational age: 32 ± 2 weeks) from the neonatal intensive care unit were included (n = 12). Infants were eligible for inclusion if they underwent a lumbar puncture for clinical indications and if informed written consent was obtained. These studies were performed in accordance with European Community Guidelines and approved by regional ethics committees. See SI Materials and Methods for more information.
Impedance Pneumography.
Infant cardiorespiratory activity was measured noninvasively using impedance pneumography and was recorded by an event monitoring system (KIDS; Hoffrichter, Schwerin, Germany). The monitor recorded baseline respiratory rates and events defined as ≥85% reduction in breathing movements for ≥10 s. The 60-s periods before and after the event were also stored in the monitor's memory.
Plethysmography After i.p. Injection of IL-1β or NaCl.
Respiration was examined using whole-body flow plethysmography in (refs. 5 and 6 in SI Materials and Methods) 9-d-old DBA/1lacJ mice (n = 143) and C57BL/6 mice (n = 16) with variable expression of mPGES-1 and EP3R, respectively. Each mouse received an i.p. injection (0.01 ml/g) of recombinant mouse IL-1β (10 μg/kg; Nordic Biosite, Täby, Sweden) or vehicle. At 70 min, the mouse was placed unrestrained into the plethysmograph chamber. Respiration was assessed during 4 min of normoxia (21% O2) followed by 1 min of hyperoxia (100% O2). After a 5-min recovery period in normoxia, the respiratory response to anoxia (100% N2) was examined. Finally, 100% O2 was administered for 8 min, and the ability to autoresuscitate was evaluated.
Plethysmography After i.c.v. Injection of PGE2 or Vehicle.
Respiration was examined using flow plethysmography in 9-d-old C57BL/6 mice (n = 38) with variable expression of EP3R. After the administration of sevoflurane anesthesia for ≈60 s, PGE2 (4 nmol in 2–4 μl of aCSF) or vehicle was slowly injected into the lateral ventricle by using a thin pulled glass pipette attached to polyethylene tubing. The mouse was then placed immediately into the plethysmograph chamber. After a 10-min recovery period in normoxia, the mouse was exposed to hyperoxic and anoxic challenge as described above. In both i.c.v. and i.p. experiments, skin temperature was measured at baseline time points through experimentation and after removal from the chamber by using thermistor temperature probe. Chamber temperature was maintained at 30.1 ± 0.1°C in accordance with the documented thermoneutral range for neonatal mice by immersing the chamber in a thermostat-controlled water bath (49).
Brainstem Respiratory Activity.
Brainstem–spinal cord preparations were rapidly isolated from 2-d-old C57BL/6 mice with EP3R+/+ and EP3R−/− genotypes as described in refs. 50 and 51 (n = 11). Respiratory-related activity corresponding to the inspiratory rhythm was monitored at the C4 ventral root through a glass suction electrode, recorded (5 kHz), and analyzed off-line. Control recordings were performed for at least 20 min before perfusion with aCSF containing PGE2 followed by an aCSF washout period.
Measurement of mPGES-1 Activity.
Newborn mouse brains (n = 33) were homogenized in 0.1 M KPi (potassium inorganic phosphate) buffer containing 0.25 M sucrose, 1× complete protease inhibitor (Roche Diagnostics, Indianapolis, IN), and 1 mM reduced glutathione followed by sonication. Membrane fraction was isolated by subcellular fractionation. mPGES-1 activity was measured in the membrane fraction as described in ref. 52.
Immunohistochemistry.
Transversal sections (14 μM) of brainstems from EP3R+/+ and EP3R−/− pups were stained with mouse monoclonal NK1R and EP3R antibodies according to standard protocols. See SI Materials and Methods for further details.
CSF Analysis and Cardiorespiratory Recordings.
CSF samples were analyzed for PGE2 and PGE2 metabolites by using a standardized enzyme immunoassay (EIA) protocol (Cayman Chemicals). Infants underwent a cardiorespiratory recording as soon as possible after the lumbar puncture (mean recording duration: 9.2 ± 2.4 h). Blood concentrations of infectious markers (e.g., C-reactive protein, white blood cells) measured within 12 h before lumbar puncture were also recorded.
Plethysmography Data Analysis.
Periods of calm respiration without movement artifact were selected for analysis. Mean fR, tidal volume (VT), and minute ventilation (VE) values during normoxia and hyperoxia as well as the anoxic response (i.e., hyperpnea, primary apnea, gasping, secondary apnea, and autoresuscitation) were analyzed as described in ref. 6. Survival was recorded for all animals. Apnea was defined as cessation of breathing for more than or equal to three respiratory cycles.
Infant Cardiorespiratory Data Analysis.
The monitoring software was used to report baseline respiratory rates and to visualize all cardiorespiratory events. The apnea index (A.I.) (number apneas per h recording) was determined. The correlation between cardiorespiratory activity, infection status, and PGE2 levels in the CSF was evaluated. All movement artifacts were excluded from analysis.
Statistics.
One-way ANOVA compared those parameters with normal distribution and equal variance. Multiple comparisons were performed using the Student's t post hoc test. Wilcoxon χ2 test was used for nonparametric measurements and data with non-Gaussian distributions. Change in variables over time was examined using multivariate ANOVA (MANOVA) repeated measures design. The Spearman's ρ correlation test determined correlations between variables. Data are presented as mean ± SEM. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Prof. Hugo Lagercrantz for his support and valuable comments; Zachi Horn, Ruth Detlofsson, and Lena Legnevall for technical assistance; and Dr. Beverly Koller (University of North Carolina, Chapel Hill, NC) for providing the C57BL/6 EP3R-deficient mice. This research was supported by Swedish Research Council Grants A0988 and 2004–5259, the Karolinska Institutet, the Swedish Society of Medicine, the Stockholm County Council, the Swedish Rheumatology Association, the Freemason Children's House, the Jerring Foundation, the Wiberg Foundation, the Tielmann Foundation, the Child Care Foundation, the Mayflower Foundation, Swedish Heart–Lung Foundation, and King Gustav V's 80 Years Foundation.
Abbreviations
- aCSF
artificial CSF
- BBB
blood–brain barrier
- COX
cyclooxygenase
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- EP3R
EP3 receptor
- fR
respiratory frequency
- i.c.v.
intracerebroventricular
- mPGES-1
microsomal prostaglandin E synthase-1
- NTS
nucleus tractus solitarius
- PGE2
prostaglandin E2
- PGH2
prostaglandin H2
- preBötC
pre-Bötzinger complex
- RVLM
rostral ventrolateral medulla.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611468104/DC1.
References
- 1.Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, Tyson JE, Philips JB, III, Edwards W, Lucey JF, et al. Pediatr Infect Dis J. 1998;17:593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Prandota J. Am J Ther. 2004;11:517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- 3.Guntheroth W. Med Hypotheses. 1989;28:121–123. doi: 10.1016/0306-9877(89)90025-x. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R. Ann NY Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 5.Olsson A, Kayhan G, Lagercrantz H, Herlenius E. Pediatr Res. 2003;54:326–331. doi: 10.1203/01.PDR.0000076665.62641.A2. [DOI] [PubMed] [Google Scholar]
- 6.Hofstetter AO, Herlenius E. Respir Physiol Neurobiol. 2005;146:135–146. doi: 10.1016/j.resp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Froen JF, Akre H, Stray-Pedersen B, Saugstad OD. Pediatrics. 2000;105:E52. doi: 10.1542/peds.105.4.e52. [DOI] [PubMed] [Google Scholar]
- 8.Lindgren C, Grogaard J. Acta Paediatr. 1996;85:798–803. doi: 10.1111/j.1651-2227.1996.tb14154.x. [DOI] [PubMed] [Google Scholar]
- 9.Stoltenberg L, Sunder T, Almaas R, Storm H, Rognum TO, Saugstad OD. J Perinat Med. 1994;22:421–432. doi: 10.1515/jpme.1994.22.5.421. [DOI] [PubMed] [Google Scholar]
- 10.Vege Å, Rognum TO, Aasen AO, Saugstad OD. Acta Paediatr. 1998;87:819–824. doi: 10.1080/080352598750013563. [DOI] [PubMed] [Google Scholar]
- 11.Ericsson A, Kovacs KJ, Sawchenko PE. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericsson A, Liu C, Hart RP, Sawchenko PE. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 13.Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 14.Coceani F, Akarsu ES. Ann NY Acad Sci. 1998;856:76–82. doi: 10.1111/j.1749-6632.1998.tb08315.x. [DOI] [PubMed] [Google Scholar]
- 15.Crestani F, Seguy F, Dantzer R. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- 16.Ericsson A, Arias C, Sawchenko PE. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra F, Savich RD, Wallen LD, Lee CH, Clyman RI, Mauray FE, Kitterman JA. J Appl Physiology. 1988;64:2160–2166. doi: 10.1152/jappl.1988.64.5.2160. [DOI] [PubMed] [Google Scholar]
- 18.Kitterman J, Liggins GC, Fewell JE, Tooley WH. J Appl Physiol. 1983;54:687–692. doi: 10.1152/jappl.1983.54.3.687. [DOI] [PubMed] [Google Scholar]
- 19.Tai TC, Adamson SL. Am J Physiol. 2000;278:1460–1473. doi: 10.1152/ajpregu.2000.278.6.R1460. [DOI] [PubMed] [Google Scholar]
- 20.Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. J Comp Neurol. 2000;428:5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. J Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballanyi K, Lalley PM, Hoch B, Richter DW. J Physiol. 1997;504:127–134. doi: 10.1111/j.1469-7793.1997.127bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliardini S, Ren J, Greer JJ. J Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitterman J, Liggins GC, Clements JA, Tooley WH. J Dev Physiol. 1979;1:453–466. [PubMed] [Google Scholar]
- 27.Guerra FA, Savich RD, Clyman RI, Kitterman JA. J Dev Physiol. 1989;11:1–6. [PubMed] [Google Scholar]
- 28.Long W. J Appl Physiol. 1988;64:409–418. doi: 10.1152/jappl.1988.64.1.409. [DOI] [PubMed] [Google Scholar]
- 29.Guerra FA, Savich RD, Wallen LD, Lee CH, Clyman RI, Mauray FE, Kitterman JA. J Appl Physiol. 1988;64:2160–2166. doi: 10.1152/jappl.1988.64.5.2160. [DOI] [PubMed] [Google Scholar]
- 30.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 31.Tai T, MacLusky NJ, Adamson SL. Brain Res. 1994;652:28–39. doi: 10.1016/0006-8993(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 32.Degi R, Bari F, Thore C, Beasley T, Thrikawala N, Busija DW. Neurobiology (Bp) 1998;6:467–468. [PubMed] [Google Scholar]
- 33.Shohami E, Gross J. J Neurochem. 1986;47:1678–1681. doi: 10.1111/j.1471-4159.1986.tb13073.x. [DOI] [PubMed] [Google Scholar]
- 34.Allen LG, Louis TM, Kopelman AE. Biol Neonate. 1982;42:8–14. doi: 10.1159/000241569. [DOI] [PubMed] [Google Scholar]
- 35.Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- 36.Degousee N, Angoulvant D, Fazel S, Stefanski E, Saha S, Iliescu K, Lindsay TF, Fish JE, Marsden PA, Li RK, et al. J Biol Chem. 2006;281:16443–16452. doi: 10.1074/jbc.M602815200. [DOI] [PubMed] [Google Scholar]
- 37.Savich RD, Guerra FA, Lee CC, Kitterman JA. J Appl Physiol. 1995;78:1477–1484. doi: 10.1152/jappl.1995.78.4.1477. [DOI] [PubMed] [Google Scholar]
- 38.Murai DT, Wallen LD, Lee CC, Clyman RI, Mauray F, Kitterman JA. J Appl Physiol. 1987;62:271–277. doi: 10.1152/jappl.1987.62.1.271. [DOI] [PubMed] [Google Scholar]
- 39.Feldman JL, Del Negro CA. 2006;7:232–241. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKay LC, Janczewski WA, Feldman JL. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- 42.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Pediatrics. 2004;113:e564–e569. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 43.Singh G, Fong LV, Salmon AP, Keeton BR. Eur Heart J. 1994;15:377–381. doi: 10.1093/oxfordjournals.eurheartj.a060506. [DOI] [PubMed] [Google Scholar]
- 44.Hoch B, Bernhard M. Acta Paediatr. 2000;89:1364–1368. doi: 10.1080/080352500300002589. [DOI] [PubMed] [Google Scholar]
- 45.Hammerman C, Zangen D. Crit Care Med. 1993;21:154–155. doi: 10.1097/00003246-199301000-00027. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 47.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, et al. Proc Natl Acad Sci USA. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming EF, Athirakul K, Oliverio MI, Key M, Goulet J, Koller BH, Coffman TM. Am J Physiol. 1998;275:F955–F961. doi: 10.1152/ajprenal.1998.275.6.F955. [DOI] [PubMed] [Google Scholar]
- 49.Jacobi MS, Thach BT. J Appl Physiol. 1989;66:2384–2390. doi: 10.1152/jappl.1989.66.5.2384. [DOI] [PubMed] [Google Scholar]
- 50.Herlenius E, Lagercrantz H, Yamamoto Y. Pediatr Res. 1997;42:46–53. doi: 10.1203/00006450-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Suzue T. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Nat Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.