Abstract
Hormone induction of growth-arrested preadipocytes triggers mitotic clonal expansion followed by expression of CCAAT/enhancer-binding protein (C/EBP)α and differentiation into adipocytes. The order of these events is critical because C/EBPα is antimitotic and its expression prematurely would block the mitotic clonal expansion required for differentiation. C/EBPβ, a transcriptional activator of the C/EBPα gene, is expressed early in the differentiation program, but lacks DNA-binding activity and fails to localize to centromeres until preadipocytes traverse the G1-S checkpoint of mitotic clonal expansion. Evidence is presented that dominant-negative CHOP-10 expressed by growth-arrested preadipocytes transiently sequesters C/EBPβ by heterodimerization. As preadipocytes reach S phase, CHOP-10 is down-regulated, apparently releasing C/EBPβ from inhibitory constraint and allowing transactivation of the C/EBPα gene. In support of these findings, up-regulation of CHOP-10 with the protease inhibitor N-acetyl-Leu-Leu-norleucinal prevents activation of C/EBPβ, expression of C/EBPα, and adipogenesis.
Keywords: 3T3-L1 preadipocyte, adipocyte, differentiation, centromere, mitotic clonal expansion
Exposure of growth-arrested preadipocytes to appropriate hormonal agents triggers a sequence of events that leads to differentiation into adipocytes. Early in the differentiation program, preadipocytes undergo several rounds of mitosis (1), i.e., mitotic clonal expansion, a process required for completion of the differentiation program (2, 3). The hormonal agents induce growth-arrested preadipocytes to synchronously enter the cell cycle and undergo mitotic clonal expansion (1, 3). Immediately after induction, and throughout mitotic clonal expansion, preadipocytes express high levels of CCAAT/enhancer-binding protein (C/EBP)β (4–6), a transcriptional activator of the C/EBPα gene (7, 8, 9). However, expression of C/EBPα is delayed until much later in the differentiation program because the preadipocytes exit mitotic clonal expansion and begin to express adipocyte genes (2, 9). The sequence of these events is critical, because C/EBPα is antimitotic and its premature expression would block the mitotic clonal expansion required for differentiation. Thus, C/EBPα appears to have dual roles in the terminal stages of adipocyte differentiation. First, C/EBPα is antimitotic (9–11) and facilitates termination of mitotic clonal expansion (2). Second, C/EBPα functions as a pleiotropic transcriptional activator of genes that produce the adipocyte phenotype. Compelling evidence has shown that expression of C/EBPα is required for adipogenesis (12, 13).
C/EBPβ is expressed immediately on induction of differentiation (6). However, at this stage of the differentiation program, C/EBPβ lacks DNA-binding activity and therefore cannot activate transcription of the C/EBPα gene. DNA-binding activity is acquired after a long lag period when preadipocytes synchronously traverse the G1-S checkpoint and initiate mitotic clonal expansion (6). Concomitant with acquisition of DNA-binding activity, C/EBPβ localizes to centromeres by binding to the multiple consensus C/EBP-binding sites in centromeric satellite DNA (6). In this paper, we provide evidence that CHOP-10, a dominant-negative member of the C/EBP family, transiently interacts with C/EBPβ, delaying acquisition of DNA-binding activity and activation of the C/EBPα gene until mitotic clonal expansion is underway.
Materials and Methods
Differentiation.
To induce differentiation, 2-day postconfluent 3T3-L1 preadipocytes (designated day 0) were fed DMEM containing 10% FBS, 1 μg of insulin per ml, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine until day 2 (6). Cells were then fed DMEM supplemented with 10% FBS and 1 μg of insulin per ml for 2 days, after which they were fed every other day with DMEM containing 10% FBS. Adipocyte gene expression and acquisition of the adipocyte phenotype begin on day 3 and are maximal by day 8.
Electrophoretic Mobility-Shift Analysis (EMSA), Western and Northern Analysis, and Immunofluorescence Microscopy.
Nuclear extracts were prepared and EMSA was performed as described (14). For mixing experiments, 28-h nuclear extract (2 μg of protein) was mixed with different amounts (2–16 μg of protein) of 0-h nuclear extract and incubated at room temperature for 30 min before EMSA. The sequence of the C/EBP regulatory element (C/EBPα gene promoter) probe for EMSA is GCG TTG CGC CAC GAT CTC TC, nucleotides 191–172.
Western analysis was performed as described (6) with rabbit anti-CHOP-10 (from David Ron, New York University Medical School), -422/aP2, -C/EBPβ, or -C/EBPα antibodies (6). For Northern analysis, equal amounts of RNA (extracted as described in ref. 15) were analyzed and probed with CHOP-10 cDNA probe (from David Ron) as described (12). Immunofluorescence microscopy was performed as described (6).
Immunoprecipitation.
Nuclear extracts were prepared as above and 1 μl of anti-C/EBPβ N-terminal antiserum was incubated with 100 μg of nuclear extract in 400 μl of 1× TTBS buffer (0.1% Tween-20/100 mM Tris·HCl, pH 7.5/0.15 M NaCl) for 1 h at room temperature, after which protein A-Sepharose was added and incubation was continued for 1 h. After centrifugation, the precipitate was washed three times with 1× TTBS, dissolved in 1× sample loading buffer, and subjected (along with the supernatant) to SDS/PAGE and Western analysis.
Results
C/EBPβ Acquires DNA-Binding Activity and Localizes to Centromeres Concomitant with Down-Regulation of CHOP-10.
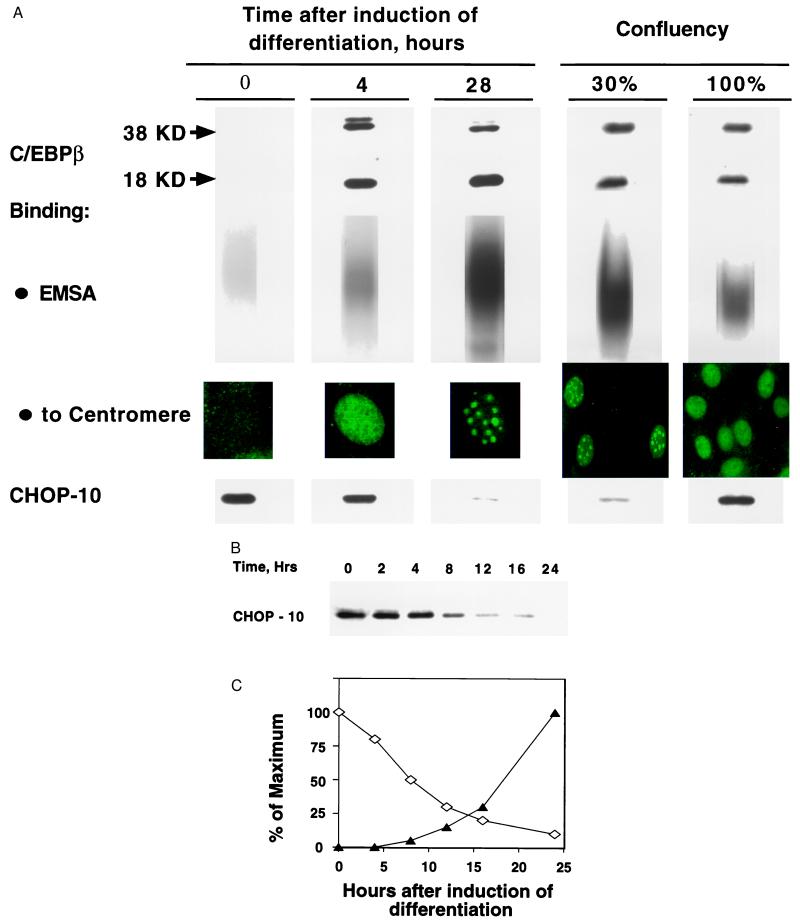
C/EBPβ is rapidly expressed (<4 h) after induction of differentiation (6). Acquisition of DNA-binding activity by C/EBPβ is delayed, however, until much later (about 14 h) when preadipocytes synchronously traverse the G1-S checkpoint at the onset of mitotic clonal expansion (6). Concomitantly, C/EBPβ localizes to centromeres. The concurrence of these and other relevant events is illustrated in Fig. 1. Maximal expression of both the 38- and 18-kDa isoforms of C/EBPβ is achieved by 4 h and persists to beyond 28 h. During this time course, the nuclear immunofluorescent staining of C/EBPβ shifts from diffuse to punctate (Fig. 1A). Previous studies (6) showed that this change in staining pattern reflects binding of C/EBPβ to satellite DNA in centromeres. As shown in Fig. 1A, these events coincide with down-regulation of CHOP-10, a dominant-negative member of the C/EBP family (16). CHOP-10 is expressed by growth-arrested (0-h) preadipocytes and is down-regulated between 4 and 28 h, the same time window during which C/EBPβ acquires DNA-binding activity. Because CHOP-10 is known to heterodimerize with members of the C/EBP family and thereby block their DNA-binding activity (16), we considered the possibility that CHOP-10 might be responsible for the blockade of DNA-binding activity by C/EBPβ early in the differentiation program.
Figure 1.
Acquisition of DNA-binding activity by C/EBPβ and concomitant down-regulation of CHOP-10 during differentiation. (A) Day 0 postconfluent 3T3-L1 preadipocytes were induced to differentiate into adipocytes by using the standard differentiation protocol. At 0, 4, and 28 h, whole-cell extracts were prepared and equal amounts of protein were separated by SDS/PAGE and subjected to immunoblotting with C-terminal antibodies against CHOP-10 and C/EBPβ. Nuclear extracts were prepared and EMSA was performed with a 32P-labeled oligonucleotide corresponding to the C/EBP regulatory element in the C/EBPα gene promoter. Preadipocytes were propagated and differentiated on cover slips, fixed, and immunostained with anti-C/EBPβ C-terminal antibody and FITC-labeled secondary antibody. To compare the proliferating and confluent states (under “Confluency”), 3T3-L1 preadipocytes were plated and allowed to proliferate to 30% or 100% confluency state, after which immunoblotting and immunostaining (with anti-C/EBPβ antibody) and EMSA was performed as above. (B) CHOP-10 levels were assessed by immunoblotting during the early stages of differentiation program. Hrs, hours after induction of differentiation. (C) Quantitation of the immunoblots shown in B, and concomitant changes in the DNA-binding activity of C/EBPβ determined EMSA by using a C/EBP regulatory element probe. ⋄, Quantitation of CHOP-10 immunoblot in B above; ▴, DNA-binding activity of C/EBPβ.
Acquisition of DNA-binding activity and centromeric localization by C/EBPβ appear to be associated with mitosis in this and certain other cell types (17, 18). Thus, both proliferating preconfluent 3T3-L1 preadipocytes (not induced to differentiate) as well as growth-arrested preadipocytes (induced to differentiate) that have reentered the cell cycle during mitotic clonal expansion exhibit these characteristics. Proliferating preconfluent preadipocytes (not induced to differentiate) express C/EBPβ that possesses DNA-binding activity and exhibits centromeric localization as evidenced by punctate immunofluorescence staining (Fig. 1A Right). However, because preadipocytes achieve confluence and exit the cell cycle, the DNA-binding activity of C/EBPβ decreases and the nuclear immunofluorescent staining becomes diffuse, despite the fact that no change in the level of C/EBPβ has occurred (Fig. 1A Right). Correlated with these changes, expression of CHOP-10 is very low in dividing preadipocytes and increases markedly as the cells become growth arrested at confluence. These findings suggest that expression of CHOP-10 and its interaction with C/EBPβ could be responsible for blocking DNA-binding activity because preadipocytes become growth-arrested at confluence.
Interaction of C/EBPβ with CHOP-10 Early in the Differentiation Program.
The possibility that CHOP-10 interacts (heterodimerizes) with C/EBPβ, thereby blocking its DNA-binding activity, was investigated. The kinetics of down-regulation of CHOP-10 and concomitant acquisition of DNA-binding activity of C/EBPβ (Fig. 1 B and C) are consistent with this premise. The level of CHOP-10 is maximal at the time of induction and then falls off over the next 16 h (Fig. 1 B and C). This decrease corresponds to the period in the differentiation program during which C/EBPβ acquires DNA-binding activity (Fig. 1C) and becomes centromere associated at the onset of mitotic clonal expansion (ref. 6 and Fig. 1A).
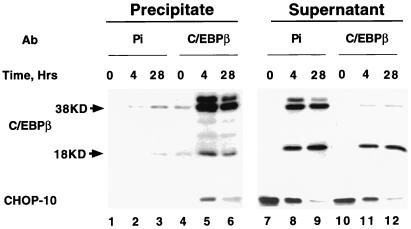
To determine whether CHOP-10 and C/EBPβ in nuclear extracts (prepared at different points in the differentiation program) interact, coimmunoprecipitation experiments were performed with rabbit antibody directed against the N-terminal region of C/EBPβ. Virtually all of the 38-kDa isoform of C/EBPβ, but only part of the 18-kDa isoform, which lacks the N-terminal epitope, was immunoprecipitated from the 4- and 28-h nuclear extract (Fig. 2 lanes 5 and 6). Thus, only part of the 18-kDa isoform heterodimerized with the 38-kDa isoform, the remainder appearing in the supernatant (lanes 11 and 12). Importantly, a large (about 45%) fraction of the CHOP-10 in the 4-h nuclear extract was immunoprecipitated with anti-N-terminal C/EBPβ antibody; by 28 h, most of the CHOP-10 had down-regulated, therefore, little was found in the supernatant (Fig. 2), so little appeared in the immunoprecipitate. It should be noted that at the 4-h time point, some CHOP-10 remained in the supernatant. It is likely that this fraction of CHOP-10 was associated with the 18-kDa isoform of C/EBPβ, both of which possesses a C-terminal Leu zipper dimerization domain with which CHOP-10 can heterodimerize (16). That immunoprecipitation by the anti-N-terminal C/EBPβ antibody is specific is indicated by the lack of immunoprecipitation of CHOP-10 at the 0-h before expression of C/EBPβ (Fig. 2, lane 4). Moreover, preimmune serum did not precipitate either C/EBPβ or CHOP-10 (Fig. 2, lanes 2 and 3). Taken together, these findings indicate that shortly after, i.e., at 4 h, induction of differentiation, but before C/EBPβ has acquired binding activity (at about 14 h after induction of differentiation), C/EBPβ is sequestered by CHOP-10.
Figure 2.
Interaction of CHOP-10 with C/EBPβ early in the differentiation program. Nuclear extracts were prepared from 3T3-L1 preadipocytes before (day 0) and at 4 and 28 h after induction of differentiation and subjected to immunoprecipitation with rabbit anti-C/EBPβ N-terminal antibody by using protein A-Sepharose. After three washes, supernatants and precipitates were separated by SDS/PAGE and immunoblotted with mouse anti-C/EBPβ C-terminal and mouse anti-CHOP-10 antibodies. Ab, antibody; Pi, preimmune serum; KD, kDa; and Hrs, hours after induction of differentiation.
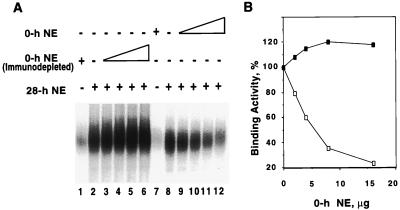
To determine whether the inability of C/EBPβ (in nuclear extract early in differentiation) to bind DNA is caused by CHOP-10, nuclear extract mixing experiments were performed. Nuclear extract from 0-h cells (before expression of C/EBPβ) that contain CHOP-10, was incubated with 28-h nuclear extract, that lacks CHOP-10 but contains “active” C/EBPβ. After incubation, binding to a consensus C/EBP-site oligonucleotide was assessed by EMSA. As shown in Fig. 3, increasing levels of 0-h nuclear extract progressively decreased binding by C/EBPβ present in 28-h nuclear extract. Proof that CHOP-10 in 0-h nuclear extract was responsible for this decreased binding activity was provided by the finding that 0-h nuclear extract, immunodepleted of CHOP-10 with anti-CHOP-10 antiserum, abolished its inhibitory activity. It should be noted that 0-h nuclear extract, preincubated with preimmune serum, retained its inhibitory activity. Taken together, these findings identify CHOP-10 as the factor in 0-h nuclear extract that sequesters C/EBPβ and blocks its DNA-binding activity early in the adipocyte differentiation program.
Figure 3.
DNA-binding activity of nuclear extract (28 h) containing C/EBPβ is sequestered by mixing nuclear extract (0 h) containing CHOP-10. (A) Nuclear extracts were prepared before (0 h) and 28 h after induction of differentiation. Nuclear extracts (2 μg of 28-h-containing C/EBPβ, Fig. 1A, and/or 2–16 μg of 0-h-containing CHOP-10, Fig. 1A) were mixed (or not) and incubated for 30 min at room temperature. Similar experiments were conducted with 0-h nuclear extract depleted of CHOP-10 by using anti-CHOP-10 antibody (immunodepleted). EMSA was then performed by using labeled C/EBP regulatory element in C/EBPα gene promoter as probe. (B) Quantitation of results in A. NE, nuclear extract. ■, Quantitation of lanes 2–6 (immunodepleted) in A; □, quantitation of lanes 8–12 in A.
Up-Regulation of CHOP-10 Blocks Acquisition of and Causes Loss of C/EBPβ-Binding Activity and the Adipocyte Phenotype.
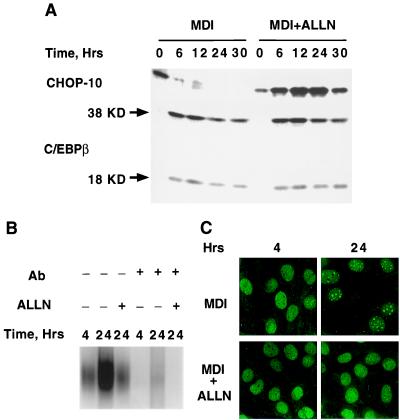
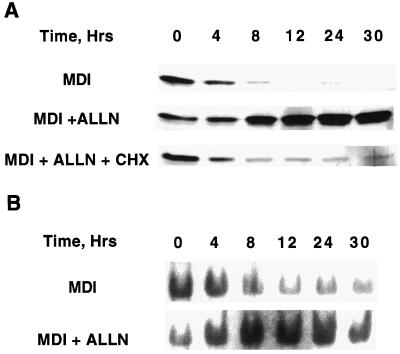
N-Acetyl-Leu-Leu-norleucine (ALLN), a calpain protease inhibitor, has been shown (19) to block acquisition of DNA-binding activity by C/EBPβ and prevent mitotic clonal expansion and adipocyte differentiation. The possibility that these effects might be caused by up-regulation of CHOP-10 was investigated. 3T3-L1 preadipocytes were induced to differentiate in the presence or absence of ALLN after which the expression of CHOP-10 was followed by Western blotting. As shown in Fig. 4A, CHOP-10 was markedly up-regulated by ALLN, whereas CHOP-10 in control cells was down-regulated normally. Although the level of C/EBPβ protein was not affected by ALLN, acquisition of DNA-binding activity by C/EBPβ was blocked (Fig. 4B). Consistent with failure to acquire DNA-binding activity, C/EBPβ did not become centromere associated (Fig. 4C). To ascertain whether the up-regulation of CHOP-10 by ALLN is caused by prevention of its degradation, preadipocytes were differentiated in the presence of both ALLN and cycloheximide to prevent additional synthesis of CHOP-10. As shown in Fig. 5A, up-regulation of CHOP-10 protein was inhibited by cycloheximide as expected; however, the cellular level of CHOP-10 was not stabilized, suggesting that degradation of CHOP-10 was not affected by ALLN.
Figure 4.
ALLN induces expression of CHOP-10 by preventing acquisition of C/EBPβ-binding activity. (A) Day 0 postconfluent 3T3-L1 preadipocytes were subjected to the standard differentiation protocol with methylisobutylxanthine/dexamethasone/insulin (MDI) with or without 26 μM ALLN. At times (Hrs) after induction of differentiation indicated, cell extracts were prepared and subjected to SDS/PAGE and immunoblotting with anti-CHOP-10 and anti-C/EBPβ antibodies; 38 and 18 KD (kDa) refer to the two isoforms of C/EBPβ. (B) Nuclear extracts were prepared from cells treated as in A and subjected to EMSA with the C/EBP-binding site probe (Fig. 1A). (C) Postconfluent 3T3-L1 preadipocytes were plated on coverslips as in Fig. 1A and induced to differentiate for 4 or 24 h in the absence or presence of ALLN, and immunofluorescence analysis was conducted as in Fig. 1A.
Figure 5.
Effect of ALLN on the turnover of CHOP-10 protein and on CHOP-10 mRNA level. (A) 3T3-L1 preadipocytes were induced to differentiate as in Fig. 1 in the absence or presence of ALLN (26 μM) with or without 10 μg/ml cycloheximide (CHX). Nuclear extracts were prepared and immunoblotted with anti-CHOP-10 antibody at the indicated times (Hrs) after induction of differentiation. (B) After induction of differentiation in the absence or presence of ALLN (26 μM), total RNA was isolated and subjected (10 μg) to Northern analysis with CHOP-10 cDNA as a probe.
To determine whether ALLN causes increased expression of CHOP-10 message, Northern analysis was performed on RNA from cells treated or not with ALLN. Fig. 5B shows that subjecting 3T3-L1 preadipocytes to the standard differentiation protocol led to the down-regulation of CHOP-10 mRNA. Induction of differentiation in the presence of ALLN, however, dramatically up-regulated CHOP-10 mRNA (Fig. 5B). Thus, it appears that ALLN induces expression of the CHOP-10 gene or prevents turnover of the CHOP-10 message.
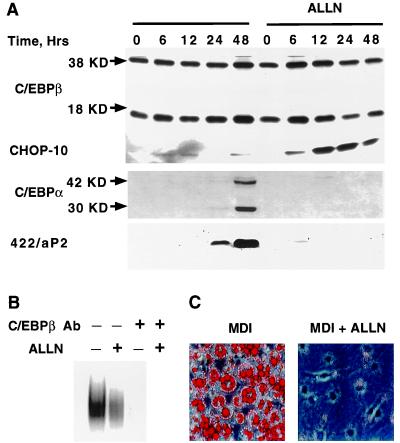
To determine whether CHOP-10 can cause loss of DNA-binding activity by C/EBPβ once it has been acquired, ALLN treatment was delayed for 20 h after induction of differentiation. At 20 h after induction, CHOP-10 has been down-regulated and C/EBPβ has acquired DNA-binding activity. As illustrated in Fig. 6A, delaying ALLN treatment until 20 h after induction caused induction of CHOP-10 expression. Under these conditions, the level of C/EBPβ remained unchanged; however, its capacity to bind DNA was drastically reduced (Fig. 6B). As would be expected, the downstream effects of decreased C/EBPβ DNA binding, i.e., expression of C/EBPα and 422/aP2 protein, were prevented (Fig. 6A) and the cells did not differentiate as indicated by failure to accumulate cytoplasmic triglyceride (Fig. 6C). These findings provide evidence that the action of CHOP-10 can both prevent acquisition of, as well as suppress, the DNA-binding activity of C/EBPβ.
Figure 6.
Effects on differentiation markers when ALLN treatment is delayed until after acquisition of DNA-binding activity by C/EBPβ. (A) 3T3-L1 preadipocytes were induced to differentiate and after 20 h were treated with ALLN (26 μM). Cell extracts were prepared at the times (Hrs) indicated and subjected to immunoblotting with anti-C/EBPβ, -C/EBPα, -CHOP-10, and -422/aP2 antibodies. (B) Cells were treated or not with ALLN as in A above. At 24 h after treatment, nuclear extracts were prepared and subjected to EMSA which was performed with C/EBP-binding site oligonucleotide (as in Fig. 4B) as probe. (C) Cells were treated or not with ALLN (for 48 h) as in A above. After treatment with ALLN (or not) for 48 h, cells were shifted to regular medium and on day 6 were fixed and cytoplasmic triglyceride stained with oil red O.
Discussion
Members of the C/EBP family of transcription factors (C/EBPβ, -δ, and -α) participate in a cascade of events (5, 6) that leads to adipocyte differentiation. These events include growth arrest followed by mitotic clonal expansion, after which C/EBPα is expressed and coordinately activates genes that give rise to the adipocyte phenotype. The sequence of these events is critical because C/EBPα, which is antimitotic, cannot precede mitotic clonal expansion. C/EBPβ is expressed early (within 4 h of induction of differentiation) in the cascade (Fig. 1 and ref. 6), but is incapable of binding DNA. Much later in the differentiation program, i.e., after the critical round of mitosis has been initiated, C/EBPβ acquires DNA-binding activity (Fig. 1 A and C and ref. 6) and transcriptionally activates the C/EBPα gene (2, 6). Presumably, acquisition of DNA-binding activity by C/EBPβ is delayed to prevent expression of C/EBPα until mitotic clonal expansion is underway, because premature expression of C/EBPα would block clonal expansion (2).
The present investigation indicates that the delay in acquisition of DNA-binding activity is caused by formation of inactive heterodimers between C/EBPβ and CHOP-10, a dominant-negative inhibitor of C/EBPs (16). CHOP-10 is expressed by growth-arrested preadipocytes and is down-regulated with kinetics consistent with the acquisition of DNA-binding activity by and centromeric localization of C/EBPβ (Fig. 1). Two lines of evidence verify the interaction between CHOP-10 and C/EBPβ: (i) antibody against the N-terminal region of C/EBPβ coimmunoprecipitates CHOP-10 from preadipocyte nuclear extracts early in the program when both CHOP-10 and C/EBPβ are expressed before C/EBPβ acquires DNA-binding activity (Fig. 1), and (ii) C/EBPβ DNA-binding activity of preadipocyte nuclear extract (that has acquired C/EBPβ DNA-binding activity and lacks CHOP-10) is inactivated when mixed with nuclear extracts from “early” preadipocytes that contain CHOP-10, but no C/EBPβ (Fig. 3). Finally, up-regulation of CHOP-10 by ALLN (a calpain inhibitor) in preadipocytes that express “active” C/EBPβ (20 h after induction of differentiation) (Fig. 6A) causes loss of DNA-binding activity by C/EBPβ (Fig. 6B) and failure to express C/EBPα and 422/aP2 and to undergo terminal differentiation into adipocytes (Fig. 6C). These findings provide compelling evidence that the cellular level CHOP-10 controls the timing of “activation” of C/EBPβ and thereby, progression of the differentiation program.
Acknowledgments
We thank Dr. David Ron (New York University Medical School) for providing the CHOP-10 cDNA and anti-CHOP-10 antibody. This work was supported by a research grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- ALLN
N-acetyl-Leu-Leu-norleucinal
- C/EBP
CCAAT/enhancer-binding protein
- EMSA
electrophoretic mobility-shift analysis
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220425597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220425597
References
- 1.Bernlohr D A, Bolanowski M A, Kelly T J, Lane M D. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- 2.MacDougald O A, Lane M D. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 3.Cornelius P, MacDougald O A, Lane M D. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Umek R M, McKnight S L. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 5.Yeh W-C, Cao Z, Classon M, McKnight S L. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q-Q, Lane M D. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Q-Q, Jiang M-S, Lane M D. Mol Cell Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christy R J, Kaestner K H, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin F-T, MacDougald O A, Diehl A M, Lane M D. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umek R M, Friedman A D, McKnight S L. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 11.Timchenko N, Wilde M, Nakanishi M, Smith J, Darlington G. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 12.Lin F-T, Lane M D. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Finegold M, Bradley A, Ou C N, Abdelsayed S V, Wilde M D, Taylor L R, Wilson D R, Darlington G J. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q Q, Jiang M S, Lane M D. Proc Natl Acad Sci USA. 1997;94:13571–13575. doi: 10.1073/pnas.94.25.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Ron D, Habener J F. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 17.Buck M, Poli V, Geer P V D, Chojkier M, Hunter T. Mol Cell. 1999;4:1087–1092. doi: 10.1016/s1097-2765(00)80237-3. [DOI] [PubMed] [Google Scholar]
- 18.Greenbaum L E, Li W, Cressman D E, Peng Y, Ciliberto G, Poli V, Taub R. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel Y M, Lane M D. Proc Natl Acad Sci USA. 1999;96:1279–1284. doi: 10.1073/pnas.96.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]