Abstract
Nature often combines independent functional domains to achieve complex function, but this approach has not been extensively explored with artificial enzymes. Here, a group I ribozyme, which can act as an endoribonuclease, was partnered with the R3C ribozyme, which catalyzes the ligation of RNA molecules. The conjoined ribozymes have the potential to perform successive RNA cleavage and joining reactions, resulting in their mutual integration into a target RNA substrate. When simply joined together, however, the ribozymes were unable to achieve this outcome because of inefficient transfer of the substrate between the two catalytic subunits. In vitro evolution was used to optimize the behavior of the conjoined ribozymes, resulting in bifunctional molecules with substantially improved integration activity. The ligase subunit of these molecules was unchanged, whereas the group I subunit acquired several mutations, mostly in peripheral regions. The generation and study of this bifunctional assembly helps shed light on the evolution of modular enzymes and the obstacles that must be overcome in bringing together independent functional domains. These molecules also may be useful as tools for the insertional mutagenesis of target mRNAs.
Modularity is a common feature in biology. On a macromolecular level, proteins often exhibit a modular architecture, being composed of independently folding domains (1, 2). Theories of exon shuffling suggest that the combination and reassortment of functional domains may have been an important mechanism for generating new activities in both protein and RNA molecules (3, 4). On a systems level, the interaction of independent functional modules can give rise to higher-level function, as exemplified by signal transduction networks and the protein-synthesizing machinery (5). Modular architectures facilitate the evolution of novel functions because functional domains are difficult to create de novo. The mixing and matching of preexisting functional units allows evolution to take advantage of a limited “tool kit” of domains.
The modular systems that exist in biology already are the product of Darwinian evolution, whereas evolution in vitro provides the opportunity to witness the development of modular systems in a controlled environment. In the laboratory, one can deconstruct the system into its component subunits and examine which features of each subunit are retained and which must adapt to allow them to function together. The motivation for generating and studying modular systems is 2-fold: first, to obtain complex function by combining independent catalytic domains; second, to understand how nature combines independent domains to achieve higher-order function.
Nucleic acid enzymes provide an attractive platform to explore questions of modularity in evolution and design. Rational design approaches have been used to construct artificial structural modules of RNA based on hairpin–loop and loop–receptor motifs that can self-assemble in a controlled fashion through tertiary interactions (6, 7). Functional RNA units also have been combined by grafting a ligand-binding domain (aptamer) onto a ribozyme to confer allosteric regulation of catalytic activity (8). The present study sought to build on these results by combining two preexisting ribozymes to generate a macromolecular complex that performs two successive reactions in a coordinated manner.
The Tetrahymena group I intron is a ribozyme that splices itself out of a precursor rRNA (9). It can be engineered to cleave an RNA substrate through a phosphoester transfer reaction that results in transfer of the 3′ portion of the substrate to the 3′ end of the ribozyme (10) (Fig. 1A). The ribozyme recognizes the substrate through Watson–Crick pairing involving an internal guide sequence located near the 5′ end of the ribozyme. The terminal guanosine 3′-hydroxyl of the ribozyme acts as the nucleophile in this reaction, which is equivalent to the reverse of the second step of splicing. In the presence of high concentrations of ligated exons and a low concentration of GTP, the ribozyme can undergo reverse splicing, inserting itself into a target RNA with concomitant release of GTP (11). This process is readily reversible, however, and the overall equilibrium favors forward splicing, consistent with the ribozyme's biological function.
Fig. 1.
Reactions of individual and conjoined ribozymes. (A) RNA cleavage catalyzed by the group I ribozyme, resulting in transfer of the 3′ portion of the substrate to the 3′ end of the ribozyme. (B) RNA ligation catalyzed by the R3C ligase ribozyme, releasing inorganic pyrophosphate. (C) Successive cleavage and ligation reactions, catalyzed by the conjoined ribozymes, resulting in their mutual integration into the RNA substrate.
The R3C ligase ribozyme was derived from a ribozyme that emerged from a library of random-sequence RNAs during an in vitro evolution experiment (12). Like the group I ribozyme, the R3C ligase binds an RNA substrate through Watson–Crick base pairing to an internal guide sequence, but in this case catalyzes attack of the terminal 3′-hydroxyl of the substrate on the α-phosphate of the 5′-triphosphate of the ribozyme, forming a 3′,5′-phosphodiester linkage and releasing inorganic pyrophosphate (Fig. 1B). The R3C ligase has a relatively simple secondary structure based on a three-way junction motif and can be pared down to an active form consisting of 70 nt.
When configured appropriately, the product of a group I-mediated cleavage reaction has the potential to act as a substrate for subsequent ligation by the R3C ribozyme. This raises the possibility of combining the two ribozymes into a single macromolecular construct in which group I-catalyzed cleavage of a target RNA substrate transfers the 3′ exon of the substrate onto the 3′ end of the construct, releasing a free 5′ exon that then is ligated onto the 5′ end of the R3C subunit (Fig. 1C). The net result would be integration of the bifunctional construct into the target RNA, analogous to reverse splicing. In this case, however, pyrophosphate release and its rapid diffusion away from the reaction site center would render the process effectively irreversible.
The biochemical challenge for two ribozymes coupled in this way is to process the RNA substrate in a coordinated manner. Both catalytic subunits must have access to the substrate, but group I-catalyzed cleavage must precede R3C-mediated ligation, and the cleaved intermediate must be retained until ligation has occurred. To achieve this, the two subunits might operate as independent catalytic domains that allow the substrate to move freely between them. Alternatively, the two modules might evolve to work in concert, translocating the substrate between the two active sites, as occurs in polyketide synthases and nonribosomal peptide synthases (13). Another possibility would be reconfiguration of the active site of one ribozyme to accommodate the activity of the other, making the subunits less modular and more integrated.
Materials and Methods
Construction of Ribozymes. DNAs encoding the conjoined ribozymes were constructed by using overlap extension PCR (14) starting with DNAs encoding the individual ribozymes. Revertants were generated by using the QuikChange kit (Stratagene), and the sequences of individual clones were confirmed. The DNAs were transcribed by using T7 RNA polymerase, and the transcribed RNAs were purified by denaturing PAGE.
In Vitro Evolution. An initial pool was generated by using mutagenic PCR (15) to amplify DNAs encoding the conjoined ribozymes, followed by in vitro transcription. The pool contained ≈5 × 1013 different RNAs, which were allowed to react with an 80-mer RNA substrate having the sequence 5′-GGACAUACGCACGACAGAACGAGAGAGCUAAAACGACUCACUAUUCCACGCACAGACUCACACAACAGACGAGCACACUC-3′ (T7 RNA polymerase promoter sequence underlined) (16). The conjoined ribozymes (0.5–1 μM) and substrate (1 μM) were preincubated separately in the presence of 1 mM GTP, 10 mM MgCl2, and 30 mM N-[2-hydroxyethyl]-piperazine-N′-[3-propanesulfonic] acid (pH 7.5) at 37°C for 10 min, then combined to initiate the reaction. The reactions were quenched with 12.5 mM EDTA, and fully integrated molecules were purified by PAGE. After elution from the gel, the molecules were amplified by RT-PCR using the One-Step RT-PCR kit (Qiagen, Valencia, CA) with primers specific to the first 21 and last 18 nt of the substrate. During some rounds of evolution, the molecules were reverse-transcribed with avian myeloblastosis virus reverse transcriptase (Amersham Pharmacia), and full-length cDNAs were purified by PAGE and subsequently amplified by PCR. A nested PCR was performed to restore the 3′ terminus by using the same forward primer and a reverse primer complementary to the last 20 nt of the group I ribozyme. This was followed by transcription and gel purification, resulting in a pool of progeny RNAs that were used to begin the next round of selection. Mutagenic PCR was performed after rounds 1 and 6, and a StEP recombination procedure (17) was performed after round 5. After round 9, individual molecules were isolated from the population by using the TA cloning kit (Invitrogen) and then sequenced and tested for their ability to integrate into a [5′-32P]-labeled substrate.
Kinetic Analysis. All reported rate constants were determined in single-turnover reactions by using trace amounts of 5′ 32P-labeled substrate and a saturating amount (1–10 μM) of either individual or conjoined ribozymes. Reactions were performed in a20–100-μl volume in the presence of 10 mM MgCl2 and 30 mM N-[2-hydroxyethyl]piperazine-N′-[3-propanesulfonic] acid at pH 7.5 and 37°C. BSA (40 μg/ml) was included to prevent adherence of RNA to the walls of the reaction vessels. For the cleavage reactions, GTP was included at 1 mM concentration. Values for kobs for the cleavage reactions were determined from a linear fit of data obtained within the first minute of the reaction. Values for kobs for the ligation reactions were determined from a curve fitted to a plot of fraction reacted versus time, based on the equation y = x(1 – e–kt), where y is the fraction reacted at time t, x is the fraction reacted at t = ∞, and k is the observed rate constant. The apparent rate constant for the overall integration reaction was obtained from the same equation based on the fraction of fully integrated product formed over several hours. The initial rate of the second step of the integration reaction was inferred by fitting data from the first 15 min of the reaction to a model of the form A → B → C, where A is unreacted substrate, B is the half-integrated intermediate, and C is fully integrated product, without constraining the amplitude of the first step. KM values were obtained from a Michaelis–Menten plot of kobs versus enzyme concentration.
Pulse–Chase Experiments. To study dissociation of exogenous 5′ exon from the R3C ligase and the evolved bifunctional RNAs, a saturating concentration of ribozyme (1–10 μM) was mixed with a trace amount of [5′-32P]-labeled 5′ exon in a 5-μl volume and allowed to bind for 2 min, reaching completion (18). The mixture was then diluted 1,000-fold in reaction buffer so that any bound substrate that subsequently dissociated would not rebind, whereas substrate that remained bound could react with the ribozyme. The dissociation rate of the 5′ exon was calculated from the equation klig = kobs + koff, where klig is the rate of ligation in the absence of a chase, kobs is the observed rate of formation of ligated product in the presence of the chase, and koff is the dissociation rate of the 5′ exon. As a control, the chase solution was premixed with the ribozyme at the start of the reaction to confirm that ligation occurred very slowly under these conditions.
To study dissociation of the 5′ exon from the group I ribozyme and conjoined ribozymes after the cleavage reaction, a saturating amount of catalyst (0.5 or 1 μM, respectively) was incubated with a trace amount of [5′-32P]-labeled substrate in a 5-μl volume and allowed to bind to completion (1 or 5 min, respectively). The reaction mixture was then diluted by 10-fold while adding 1 μM unlabeled substrate and 10 μM R3C ligase, and ligation of any released 5′ exon to the added R3C ligase was monitored over time. For the group I ribozyme, the rate of dissociation of the 5′ exon was obtained directly from the rate of ligation to R3C molecules present in the chase solution. For the conjoined ribozymes, the ratio between dissociation and ligation rate constants was assessed qualitatively by comparing the amount of fully integrated product to the amount of ligated product formed during the chase.
Results
Rationally Designed Constructs. As a preliminary test, the two independent ribozymes were incubated in the same reaction vessel with a common substrate. Cleavage of the substrate by the group I ribozyme was observed, but subsequent ligation to the R3C ribozyme occurred at a rate of only ≈10–3 min–1, consistent with literature values for the rate of dissociation of the 5′ exon product from the group I ribozyme (19, 20). When the group I cleavage product was purified and presented to the R3C ligase in a separate reaction mixture, ligation occurred rapidly, suggesting that the cleavage product was sequestered by the group I ribozyme in the common reaction mixture. This hypothesis was confirmed by pulse–chase experiments. Variants of the group I ribozyme that formed partial mismatches with the substrate or contained mutations known to lower its affinity for the product (21–23) exhibited more efficient progression from the cleavage reaction to the ligation reaction.
The two ribozymes were then joined in various arrangements that might serve as a starting point for evolutionary optimization of their combined integration activity. Two classes of conjoined molecules were synthesized: dual P1 constructs composed of the two ribozymes, each with their own substrate-binding site and tethered by an oligonucleotide linker, and single P1 constructs composed of the two ribozymes joined by a common substrate-binding site. The two different forms of conjoined molecules face different biochemical hurdles to carry out integration. For the dual P1 molecules, the substrate must dissociate from the group I subunit after cleavage and subsequently bind to the R3C subunit of the same molecule. For the single P1 molecules, the substrate either could translocate between the two subunits without dissociating or both reactions could be carried out without changing the position of the substrate.
Both classes of constructs were evaluated for their ability to become integrated into an RNA substrate in a manner that depended on the release of inorganic pyrophosphate from the 5′ end of the R3C subunit. This was assessed by comparing the activity of triphosphate-bearing and dephosphorylated ribozymes. Although some single P1 constructs exhibited a small amount of triphosphate-dependent integration activity, a substantial amount of shorter-length side products were observed. These shorter products may be the result of either an autocyclization reaction or two successive phosphoester transfer reactions mediated by the group I subunit. In contrast, the dual P1 constructs exhibited no appreciable integration activity. Both classes of constructs had substantial cleavage activity, attributable to the group I subunit, but only a small fraction of the released 5′ exon became ligated to the R3C subunit. Thus, it was necessary to use in vitro evolution to improve the efficiency and coordinated activity of the conjoined ribozymes.
In Vitro Evolution. The selection scheme relied on the acquisition of novel sequence tags at both the 5′ and 3′ ends to distinguish fully integrated molecules from those that were either unreacted or only partially integrated. Fully integrated molecules were purified based on their mobility in a denaturing polyacrylamide gel, then subjected to selective RT-PCR using primers specific to the substrate sequences flanking the site of integration. A subsequent nested PCR amplification was carried out to restore the original 3′ end, followed by in vitro transcription to generate a pool of molecules to begin the next round of selective amplification. GTP was included in the reaction mixture during most rounds to disfavor reverse splicing catalyzed by the group I subunit alone.
Nine rounds of in vitro evolution were carried out, using mutagenized pools of both the dual and single P1 constructs. No significant amount of reacted products were observed in the lineage based on the dual P1 construct. For the single P1 lineage, however, there was substantial enrichment of the ability to generate what seemed to be a full-length integrated product. Beginning in the fourth round of evolution, the reaction time was decreased progressively from 4 h to 15 s to favor the emergence of more efficient catalysts (24). After nine rounds, individual molecules from the single P1 lineage were cloned and analyzed.
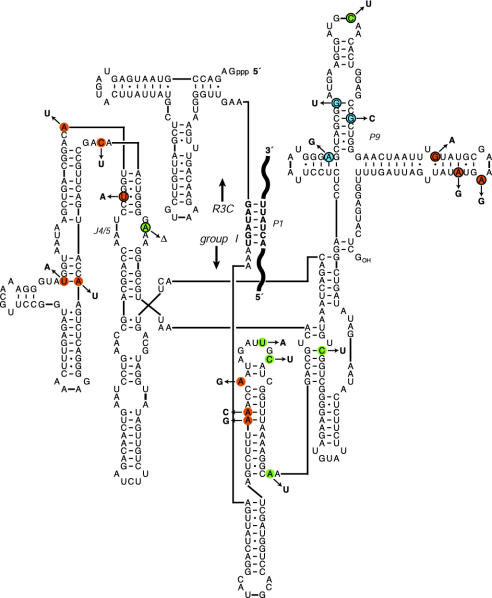
Individuals isolated from the final selected population varied significantly in size, transcription efficiency, and integration activity. Fourteen cloned individuals were assayed for their ability to react with a [5′-32P]-labeled substrate in the presence of GTP, and two in particular exhibited substantially improved activity compared with the starting single P1 construct. Sequence analysis of these two individuals, referred to as clones 9-3 and 9-13, revealed that they contained 11 and 14 mutations, respectively (Fig. 2). Strikingly, none of the mutations occurred within the R3C ligase subunit, even though the cumulative frequency of mutation was ≈2% per nucleotide position.
Fig. 2.
Secondary structure of the evolved bifunctional RNA molecules. Residues that were mutated in the 9-3 RNA are shown in green, those that were mutated in the 9-13 RNA are shown in red, and those that were mutated in both evolved molecules are shown in blue. The J4/5 and P9 regions are indicated, and the mutations within these regions that were reverted are outlined with a black circle.
Fig. 3 shows the reaction products and time course of the integration reaction catalyzed by either the triphosphate-bearing or dephosphorylated 9-13 RNA. As expected of bifunctional molecules that perform the desired cleavage and ligation reactions, the presence of the 5′-triphosphate was essential for integration. The dephosphorylated 9-13 RNA generated a substantial amount of the free 5′ exon, but almost no integrated product. The small amount of integrated product that was observed with the dephosphorylated RNA may be caused by either incomplete dephosphorylation or two successive group-I-mediated phosphoester transfer reactions involving attack at a guanosine residue near the 5′ end of the R3C subunit.
Fig. 3.
Integration activity of the evolved 9-13 RNA. (A) Comparative activity of the starting construct (wt) and either the dephosphorylated or 5′-triphosphorylated 9-13 RNA. The RNA (5 μM) was incubated for 2 h in the presence of a trace amount of [5′-32P]-labeled substrate and 10 mM MgCl2 and either the presence or absence of 1 mM GTP, at pH 7.5 and 37°C. The marker lane at the right shows the 5′ exon and the integrated product. Asterisks indicate misintegrated and shorter length cleavage products resulting from reactions catalyzed by the group I subunit alone, seen most prominently for the starting construct. (B) Time course of integration activity of either dephosphorylated or 5′-triphosphorylated 9-13 RNA, incubated with [5′-32P]-labeled substrate in the absence of GTP under the same reaction conditions as above. Reaction of the triphosphorylated RNA gave rise to free 5′ exon (○) and a larger amount of fully integrated product (•). Reaction of the dephosphorylated RNA gave rise to a large amount of free 5′ exon (□) and a small amount of integrated products (▪).
The presumed integrated product was isolated by PAGE, amplified by RT-PCR, and sequenced, confirming its precise integration at the expected location. The 5′-terminal guanosine was retained in the integrated product, demonstrating that the majority of this material was not the result of group I-mediated reverse splicing. When the purified integrated product was incubated in reaction buffer in either the presence or absence of GTP, the observed rate of its breakdown caused by either uncatalyzed or catalyzed cleavage events was at least 10-fold slower than the rate of formation of the integrated product.
In addition to the expected reaction intermediate and fully integrated product, several side products accumulated over time. Some of these were caused by miscleavage by the group I subunit at nucleotide positions upstream of the expected integration site. In addition, some of the released full-length 5′ exon seemed to become ligated to a different RNA molecule that had not undergone the first step of integration, giving rise to a product that contained the 5′ exon but not the 3′ exon. As expected, dephosphorylation of the RNA greatly reduced formation of this 5′ exon-containing product. In the absence of GTP, the released 5′ exon can either ligate to the 5′ end of the bifunctional RNA in an R3C-mediated reaction or undergo a phosphoester transfer reaction after an internal guanosine residue in a group I-mediated reaction. The latter is equivalent to the reverse of the first step of group I splicing and, depending on the particular guanosine residue involved, could generate a variety of reaction products. These observed side products were slightly smaller in size compared with the partially and fully integrated products and were not formed when GTP was present in the reaction mixture.
Catalytic Properties of the Evolved RNAs. The constellation of mutations that occurred in the evolved 9-3 and 9-13 RNAs resulted in substantial improvement in integration activity compared with that of the rationally designed conjoined molecule. To discern which steps of the reaction pathway were affected, kinetic analyses were performed comparing the behavior of the starting and evolved conjoined ribozymes and the two isolated ribozyme domains (Fig. 4). The two evolved RNAs generated fully integrated product at an apparent rate of ≈10–2 min–1. As expected of a two-step reaction, there was a lag between release of the 5′ exon intermediate and subsequent ligation to give the fully integrated product (25). The initial rate of substrate cleavage by the 9-13 RNA was 0.01 min–1. This was ≈50-fold slower than the rate of cleavage observed for the starting construct or the isolated WT group I ribozyme, which was 0.7 or 0.4 min–1, respectively. Fitting both the overall rate and the rate of the first step to a two-step reaction model gave an initial rate for the ligation step of 0.07 min–1. Under conditions of excess 9-13 RNA, the amount of labeled substrate that became converted into fully integrated product reached a plateau of ≈15%. Similar kinetic behavior was observed for the 9-3 RNA.
Fig. 4.
Catalytic rates of the starting and evolved conjoined ribozymes and the two isolated ribozyme subunits. (A) RNA cleavage catalyzed by the group I ribozyme. (B) RNA ligation catalyzed by the R3C ribozyme, which did not acquire any mutations during the course of evolution. (C) First and second steps of integration catalyzed by the conjoined ribozymes. The WT construct produced almost no integrated product. (D) Ligation of exogenously supplied 5′ exon by the conjoined ribozymes. Values for kobs are in units of min–1, shown for the 9-13 evolved RNA. Similar values were obtained for the 9-3 RNA.
The evolved bifunctional molecules performed the initial cleavage step slower than the starting conjoined molecule, but once the evolved molecules carried out cleavage they underwent rapid ligation to give the integrated product. The slower cleavage rate of the evolved molecules may be caused by a defect in folding, substrate binding, or catalysis. Separating the evolved molecules into their constituent catalytic subunits revealed that the isolated group I domain cleaved the substrate rapidly (Fig. 4A), although the evolved forms of this domain had a greater tendency to miscleave the substrate compared with the WT group I ribozyme. This finding suggests that the presence of the R3C subunit somehow interferes with the cleavage activity of the evolved molecules.
One potential strategy for improving the efficiency of transfer of the 5′ exon intermediate from the active site of the group I subunit to that of the R3C subunit would be to increase the rate of dissociation of the 5′ exon from the group I subunit. To test this hypothesis, pulse–chase experiments were performed with either the WT group I ribozyme or the evolved group I domain in isolation. The ribozyme first was allowed to bind a trace amount of 5′-labeled substrate; the reaction was then chased by 10-fold dilution and addition of 1 μM unlabeled substrate and 10 μM R3C ligase. The large excess of added R3C ligase served to capture any 5′ exon intermediate generated by group I-mediated cleavage that dissociated from the group I ribozyme during the chase period. This allowed the rate of dissociation to be measured, provided that dissociation from the group I domain was slower than the rate of ligation to the chase ribozyme.
The rate of dissociation of the 5′ exon from both the WT group I ribozyme and the evolved group I domain was indeed slow, with an upper limit of 10–3 min–1, which is in good agreement with previous measurements of the rate of product dissociation from the WT group I ribozyme (19, 20). Analogous experiments using the evolved 9-3 RNA revealed ≈100-fold faster dissociation of the 5′ exon after the cleavage step at a rate approximately equal to the rate of 5′ exon ligation to the R3C subunit. Thus, the presence of the R3C subunit substantially affects product release by the adjoining group I subunit. The results were not affected by increasing the concentration of R3C ligase in the chase solution. It was not possible to measure dissociation of the 5′ exon from the starting conjoined molecule because of rapid miscleavage that gave rise to 3′-truncated forms of the 5′ exon.
When exogenous 5′ exon was supplied to the starting conjoined molecule, mimicking the second step of the integration pathway, almost no ligation occurred. The 9-13 RNA, however, ligated the exogenous 5′ exon at a rate of 0.2 min–1 (Fig. 4D), which is similar to the rate observed for the isolated R3C ligase domain (0.3 min–1) and similar to the inferred rate of the second step of the integration pathway (0.07 min–1). This finding implies the absence of a rate-limiting step of substrate transfer between the two ribozyme subunits. Analysis of the concentration dependence of the ligation reaction revealed that the KM of the isolated R3C ligase for the 5′ exon substrate is ≈2 μM. This is similar to the KD of ≈13 μM that would be predicted based on Watson–Crick pairing between the ribozyme and substrate (26). The evolved conjoined ribozymes, however, exhibited a ≈20-fold improvement in KM for this reaction, to ≈100 nM.
The KM for the ligation reaction could reflect the KD for substrate binding or a combination of association and dissociation rates and catalytic rate constants. Pulse–chase experiments were conducted to clarify this issue (18). These experiments revealed that the substrate dissociates from the isolated R3C ribozyme much faster than the rate of ligation, but that for the evolved bifunctional RNAs substrate dissociation and ligation occur at similar rates. Because no mutations were present in the R3C subunit of the evolved molecules, these data suggest that binding interactions derived from the group I subunit help to retain the 5′ exon intermediate for subsequent ligation catalyzed by the R3C subunit.
To assess the contribution of particular mutations to the improved catalytic behavior of the evolved molecules, clusters of these mutations were reverted to the WT in both the 9-3 and 9-13 RNAs, and the effect on catalytic activity was studied. P9 is a peripheral element of the group I ribozyme and was the location of many of the mutations observed in both evolved molecules (Fig. 2). When the P9 mutations were reverted, almost no fully integrated product was observed. The P9 revertants still cleaved the substrate to generate free 5′ exon, albeit at a slower rate, but the free 5′ exon dissociated rapidly from the molecule that had generated it; then it became bound and ligated to a different molecule. The P9 revertants exhibited a ≈20-fold higher KM for the ligation reaction compared with the evolved molecules that contained the P9 mutations. Pulse–chase experiments indicated that the 5′ exon intermediate dissociated more rapidly from the P9 revertants than from the evolved molecules, accounting for at least part of this effect. The J4/5 region of the group I ribozyme is known to play a key role in binding the substrate P1 duplex (27–29). Both the 9-3 and 9-13 evolved molecules contain mutations in or adjacent to the J4/5 region (Fig. 2). When these mutations were reverted to WT, the resulting molecules exhibited a faster rate of substrate cleavage but a similar overall rate of integration compared with the evolved molecules.
Discussion
A rationally designed construct linking the group I ribozyme and R3C ligase integrates with very low efficiency into a target RNA substrate. In vitro evolution was carried out to obtain a substantial increase in this activity. Integration is achieved through sequence-specific endonucleolytic cleavage of an RNA substrate followed by RNA ligation. The two steps involve a different chemical mechanism and require the coordination of successive binding events and multiple functional interactions. Such complex behavior might be difficult to realize through the optimization of a single catalytic RNA domain.
During in vitro evolution, both the group I and R3C subunits had the opportunity to mutate, but all of the mutations that were present in the final evolved molecules occurred within the group I subunit. Both subunits displayed significantly different behavior when coupled together compared with their behavior in isolation. The evolved group I subunit in isolation cleaved the substrate more rapidly and had a much greater tendency to cleave at an incorrect position compared with the WT group I ribozyme. Addition of the R3C ligase subunit to the evolved group I subunit resulted in slower cleavage and much faster dissociation of the 5′ exon intermediate. The appended R3C ligase subunit readily captured and ligated the 5′ exon, completing the integration process. The 5′ exon bound more tightly to the conjoined ribozymes compared with the R3C ribozyme in isolation, favoring completion of the integration process.
The J4/5 region of the group I ribozyme has been shown to make tertiary contacts with the P1 substrate duplex, forming the side of a binding crevice into which the duplex is docked (27–29). Both the 9-3 and 9-13 evolved RNAs contain mutations in or adjacent to the J4/5 region (Fig. 2). Reverting these mutations to the WT sequence resulted in a faster rate of substrate cleavage but a slower rate of ligation, resulting in greater accumulation of the 5′ exon intermediate compared with what was observed for the evolved molecules. Thus, the mutations in the J4/5 region seem to facilitate transfer of the 5′ exon intermediate between the two subunits.
The P9 region of the group I ribozyme is a peripheral domain that is not essential for catalytic activity (30). Both the 9-3 and 9-13 evolved RNAs contain several mutations in the P9 region, including three mutations that are common to both molecules. Reversion of these mutations to the WT sequence resulted in almost no fully integrated product being formed. The P9 mutations seem to play multiple roles; some may facilitate group I cleavage in the presence of the J4/5 mutations, whereas others may contribute to binding of the 5′ exon intermediate by the R3C ligase subunit.
During the evolution process, the conjoined ribozymes were selected for the ability to transfer the substrate between the two active sites in an efficient manner. In seeking to understand how they met this challenge, one must consider the evolutionary heritage of the two component subunits. The group I ribozyme was evolved to perform a self-splicing reaction. During splicing, it binds the 5′ exon intermediate with a KD of 15 pM, which is 1,000-fold tighter than would be expected based on Watson–Crick pairing alone (20, 26). The 5′ exon intermediate is bound much more tightly than the ligated exons (31, 32), reflecting selection pressure favoring reliable progression from the first to the second step of splicing (21). The R3C ligase, in contrast, was derived from an in vitro evolution experiment in which a pool of random-sequence RNAs were challenged to ligate an excess of RNA substrate. In keeping with these selection pressures, the group I ribozyme reacts almost every time it binds substrate (19), whereas the R3C ligase releases the substrate more quickly than it reacts. For the evolved bifunctional molecules, the 5′ exon intermediate dissociates at about the same rate that it ligates to the R3C subunit.
Evolving biological systems must balance the robustness of individual catalysts against their ability to operate together as part of a functional pathway. The mutations that were selected in this study, while impairing the cleavage activity of the group I ribozyme, allowed it to accommodate another functional domain to achieve improved overall behavior. All of the mutations in the evolved RNAs were located within the group I subunit. This subunit contains 393 nt, of which only ≈25% are essential for catalytic activity (33). In contrast, the 70-nt R3C subunit was based on a minimized version of a ligase ribozyme that contains few extraneous nucleotides and thus may have been more functionally constrained.
Unlike naturally occurring mobile introns and transposable elements, which splice themselves both into and out of target sequences, the evolved molecules developed in this study are driven toward insertion by the release of inorganic pyrophosphate during the ligation step. Combining two ribozyme subunits to achieve integration into a target RNA in an essentially irreversible manner may have practical applications for modifying biological RNA sequences. With further development, these bifunctional molecules might be used to deliver exogenous coding sequences into natural mRNAs, thereby generating fusion proteins in a native context. This could provide a useful tool for probing cellular function and altering the localization or stability of particular proteins.
Acknowledgments
We thank Martha Fedor, James Williamson, Plachikkat Radha, and members of the Joyce laboratory for helpful discussions. This work was supported by the National Institutes of Health and a Skaggs Predoctoral Fellowship (to R.M.K.).
References
- 1.Bork, P. (1992) Curr. Opin. Struct. Biol. 2, 413–421. [Google Scholar]
- 2.Voigt, C. A., Martinez, C., Wang, Z. G., Mayo, S. L. & Arnold, F. H. (2002) Nat. Struct. Biol. 9, 553–558. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert, W. (1978) Nature 271, 501. [DOI] [PubMed] [Google Scholar]
- 4.Westhof, E., Masquida, B. & Jaeger, L. (1996) Folding Des. 1, 78–88. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell, L. H., Hopfield, J. J., Leibler, S. & Murray, A. W. (1999) Nature 402, 47–52. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger, L. & Leontis, N. B. (2000) Angew. Chem. 39, 2521–2524. [DOI] [PubMed] [Google Scholar]
- 7.Jaeger, L., Westhof, E. & Leontis, N. B. (2001) Nucleic Acids Res. 29, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soukup, G. A. & Breaker, R. R. (2000) Curr. Opin. Struct. Biol. 10, 318–325. [DOI] [PubMed] [Google Scholar]
- 9.Kruger, K., Grabowski, P. J., Zaug, A. J., Sands, J., Gottschling, D. E. & Cech, T. R. (1982) Cell 31, 147–157. [DOI] [PubMed] [Google Scholar]
- 10.Zaug, A. J., Been, M. D. & Cech, T. R. (1986) Nature 324, 429–433. [DOI] [PubMed] [Google Scholar]
- 11.Woodson, S. A. & Cech, T. R. (1989) Cell 57, 335–345. [DOI] [PubMed] [Google Scholar]
- 12.Rogers, J. & Joyce, G. F. (2001) RNA 7, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosla, C. & Harbury, P. B. (2001) Nature 409, 247–252. [DOI] [PubMed] [Google Scholar]
- 14.Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. & Pease, L. R. (1989) Gene 77, 61–68. [DOI] [PubMed] [Google Scholar]
- 15.Cadwell, R. C. & Joyce, G. F. (1994) PCR Methods Appl. 3, S136–S140. [DOI] [PubMed] [Google Scholar]
- 16.Wright, M. C. & Joyce, G. F. (1997) Science 276, 614–617. [DOI] [PubMed] [Google Scholar]
- 17.Zhao, H., Giver, L., Shao, Z., Affholter, J. A. & Arnold, F. H. (1998) Nat. Biotechnol. 16, 258–261. [DOI] [PubMed] [Google Scholar]
- 18.Stage-Zimmermann, T. K. & Uhlenbeck, O. C. (1998) RNA 4, 875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herschlag, D. & Cech, T. R. (1990) Biochemistry 29, 10159–10171. [DOI] [PubMed] [Google Scholar]
- 20.Karbstein, K., Carroll, K. S. & Herschlag, D. (2002) Biochemistry 41, 11171–11183. [DOI] [PubMed] [Google Scholar]
- 21.Herschlag, D. & Cech, T. R. (1990) Biochemistry 29, 10172–10180. [DOI] [PubMed] [Google Scholar]
- 22.Young, B., Herschlag, D. & Cech, T. R. (1991) Cell 67, 1007–1019. [DOI] [PubMed] [Google Scholar]
- 23.Zarrinkar, P. P. & Sullenger, B. A. (1999) Biochemistry 38, 3426–3432. [DOI] [PubMed] [Google Scholar]
- 24.Tsang, J. & Joyce, G. F. (1994) Biochemistry 33, 5966–5973. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, K. A. (1992) in The Enzymes, ed. Sigman, D. S. (Academic, San Diego), Vol. XX, pp. 1–61. [Google Scholar]
- 26.Zuker, M., Mathews, D. H. & Turner, D. H. (1999) in RNA Biochemistry and Biotechnology, eds. Barciszewski, J. & Clark, B. F. C. (Kluwer, Dordrecht, The Netherlands), pp. 11–43.
- 27.Strobel, S. A., Ortoleva–Donnelly, L., Ryder, S. P., Cate, J. H. & Moncoeur, E. (1998) Nat. Struct. Biol. 5, 60–66. [DOI] [PubMed] [Google Scholar]
- 28.Wang, J. F., Downs, W. D. & Cech, T. R. (1993) Science 260, 504–508. [DOI] [PubMed] [Google Scholar]
- 29.Cate, J. H., Gooding, A. R., Podell, E., Zhou, K., Golden, B. L., Kundrot, C. E., Cech, T. R. & Doudna, J. A. (1996) Science 273, 1678–1685. [DOI] [PubMed] [Google Scholar]
- 30.Joyce, G. F. & Inoue, T. (1987) Nucleic Acids Res. 15, 9825–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei, R. & Herschlag, D. (1996) Biochemistry 35, 5796–5809. [DOI] [PubMed] [Google Scholar]
- 32.Emerick, V. L., Pan, J. & Woodson, S. A. (1996) Biochemistry 35, 13469–13477. [DOI] [PubMed] [Google Scholar]
- 33.Beaudry, A. A. & Joyce, G. F. (1990) Biochemistry 29, 6534–6539. [DOI] [PubMed] [Google Scholar]