Abstract
While the term neuroinflammation often conjures up images of cellular damage, mounting evidence suggests that certain proinflammatory molecules, such as the cytokine IL-1β, may have beneficial and protective effects. In a report in this issue of the JCI, Shaftel and coworkers have generated an elegant mouse model in which local hippocampal overexpression of IL-1β in an Alzheimer disease (AD) transgenic mouse model resulted not in the expected exacerbation of the amyloid β plaque deposition common to AD, but instead in plaque amelioration (see the related article beginning on page 1595). Thus, manipulation of the immune system may be a potential therapeutic approach to protect against AD, although further studies are needed to understand all of the downstream effects of this manipulation.
The role of neuroinflammation in Alzheimer disease (AD) remains somewhat of a mystery. Although inflammatory proteins such as cytokines, chemokines, complement, and acute phase proteins are elevated in AD brain, the status of each as a “good guy” or a “bad guy” is still unclear (1). On one hand, inflammation may be a secondary response to progressive neurodegeneration in the brain. In this instance, immune cells in the brain, such as microglia and astrocytes, may become activated and secrete inflammatory molecules that may then further accelerate pathogenesis (2). On the other hand, recent evidence points to a potentially beneficial and protective role of inflammation in the brain (3). In this case, inflammation appears to induce microglial phagocytosis of amyloid β (Aβ) protein, reducing its deposition into plaques. Aβ protein is proteolytically cleaved from its large precursor, amyloid precursor protein (APP), and secreted; in AD, Aβ accumulates extracellularly into plaques, a hallmark pathological feature of the disease (4).
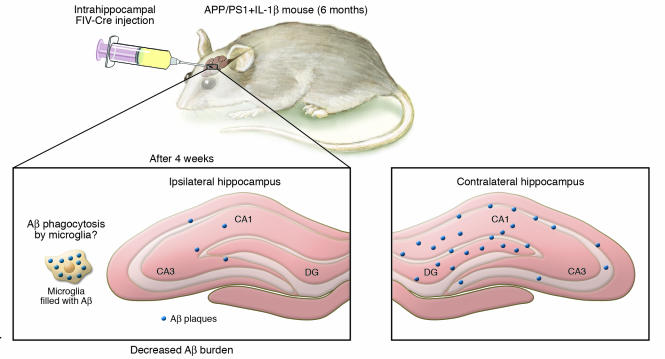
In a study reported in this issue of the JCI, Shaftel and coworkers present provocative new findings in support of the latter scenario (5). The authors overexpressed the proinflammatory cytokine IL-1β (IL-1β) in a region- and time-dependent fashion in transgenic mice overexpressing human mutant APP and presenilin 1 (APP/PS1 mice), a mouse model designed to partially mimic AD pathology in which extracellular Aβ plaques accumulate in the brain with aging. Excisional activation transgene (XAT) technology was used to first generate IL-1βXAT transgenic mice (5). Upon injection of feline immunodeficiency virus–Cre (FIV-Cre) to locally activate the IL-1β transgene in brain, these mice were shown to have elevated levels of IL-1β and glial fibrillary acid protein (GFAP), indicative of astrocytic activation, as well as of ionized calcium-binding adaptor molecule 1 (Iba-1) and MHC class II, indicative of microglial activation. Expression of the transgene was still apparent 1 year after injection. These mice were then crossbred with APP/PS1 mice, and the resulting mice expressing the APP/PS1 and IL-1βXAT transgenes (APP/PS1+IL-β mice) were injected with FIV-Cre to induce IL-1β overexpression specifically in the hippocampus at 6 months of age, during early Aβ plaque deposition (Figure 1). To the authors’ surprise, instead of observing a worsening of AD-like pathogenesis 4 weeks after injection as expected, the opposite effect occurred. IL-1β overexpression in the APP/PS1+IL-1β mouse hippocampus caused reduced Aβ plaque deposition and lower insoluble Aβ levels in the injected ipsilateral hippocampus compared with the control-injected contralateral hippocampus in individual mice. The injected hippocampi also had fewer Aβ deposits than those found in age-matched APP/PS1 mice. No changes were observed in the levels of APP or β-secretase, the latter being one of the enzymes responsible for cleaving Aβ from APP, which suggests that the reduction in plaque burden was not the result of a decline in Aβ production. Instead, the focal overexpression of IL-1β led to a large increase in astrocytic activation (reflected by increased levels of GFAP) and microglial activation (reflected by increased levels of microglial markers Iba-1 and MHC class II) in the injected hippocampi compared with the contralateral hippocampi in APP/PS1+IL-1β mice.
Figure 1. Overexpression of IL-1β activates glial cells and reduces Aβ plaque deposition in a mouse model of AD.
In this issue of the JCI, Shaftel and colleagues (5) used XAT technology to generate IL-1βXAT transgenic mice and crossbred these mice with APP/PS1 mice, a mouse model used to study AD. Intrahippocampal injection of FIV-Cre at 6 months of age locally activated the IL-1β transgene and increased the levels of IL-1β and glial fibrillary acid protein (GFAP), indicative of astrocytic activation, as well as Iba-1 and MHC class II, indicative of microglial activation. One month later, instead of observing a worsening of AD-like pathogenesis as expected, the authors found that IL-1β overexpression in the APP/PS1+IL-1β mouse resulted in reduced Aβ plaque deposition and lower insoluble Aβ levels in the injected ipsilateral hippocampus compared with the control-injected contralateral hippocampus. Levels of APP and β-secretase, one of the APP-cleaving enzymes responsible for generating Aβ, were unchanged, suggesting that Aβ production was not affected. Instead, IL-1β overexpression led to increased microglial activation and possibly, as the authors hypothesize, clearance of Aβ deposits by microglial phagocytosis. CA, cornu ammonis; DG, dentate gyrus.
The authors conclude that IL-1β upregulation in AD brain may be an attempt to stimulate microglia to phagocytose Aβ deposits, and thus may have a positive role in AD brain. The authors suggest that antiinflammatory treatment may suppress the ability of IL-1β, and possibly other “good” cytokines, to induce clearance of Aβ via microglial phagocytosis and should therefore be used with caution in AD patients.
Inflammation and neurodegeneration
The potential beneficial effects of inflammation and immune cell activation are areas of growing interest in AD research. Evidence for Aβ plaque–lowering effects have been demonstrated in APP transgenic mice overexpressing the cytokine TGF-β (6), in APP transgenic mice treated with glatiramer acetate and a proteosome-based adjuvant (7), in acute experiments in APP/PS1 mice injected intracranially with LPS (1), and in APP transgenic mice treated with antiinflammatory drugs (8, 9). Interestingly, chronic LPS treatment resulted in increased plaque deposition, suggesting that inflammation may have different effects depending on its duration (1). In support of a beneficial role for inflammation in AD, suppression of certain inflammatory molecules has been found to worsen AD pathogenesis. For example, inhibition of complement C3 by overexpression of the C3 inhibitor soluble complement receptor–related protein Y (sCrry) (10) or by genetic ablation (i.e., C3-null; author’s unpublished observations) in APP transgenic mice led to an age-dependent increase in cortical and hippocampal plaque burden and neurodegeneration. In addition, suppression of microglial activation, either by minocycline treatment (11) or by CC chemokine receptor 2 (Ccr2) deficiency (12), in young APP transgenic mice resulted in increased cerebral Aβ deposition.
However, as was pointed out in an excellent recent review of inflammation in AD (3), inflammatory molecules are multifunctional and, depending on timing and context, can produce either pro- or antiinflammatory effects. This may be particularly relevant for IL-1β. For example, IL-1β gene expression (13) and protein levels (14) are elevated in human AD brain tissue. If IL-1β were protective, one would expect a lowering of Aβ levels and less neurodegeneration. It is possible that the immune system becomes overwhelmed with Aβ deposition to the point where microglial activation by IL-1β is no longer effective. Interestingly, a recent report demonstrated that Aβ deposition and Aβ clearance via passive Aβ immunotherapy were unchanged in APP transgenic mice crossbred with IL-1 receptor 1 (IL-1R1) knockout mice, suggesting that IL-1 signaling (e.g., IL-1β binding to IL-1R1) may not be required for microglial phagocytosis of Aβ plaques, although it is also possible that other IL-1 receptors may compensate for the lack of IL-1R1 (15). In addition, it has been reported that IL-1β has other effects in the brain, such as promoting tau phosphorylation (16, 17). Neurofibrillary tangles, another pathological hallmark of AD, are the result of tau hyperphosphorylation and paired helical filament formation inside neurons that cause disruption of neuronal function (4).
It will be important to determine the effects of local IL-1β overexpression on vascular amyloid level, neuronal pathology (including tau phosphorylation), and cognition in the mouse model described by Shaftel and colleagues (5) as well as how these changes and IL-1β elevation affect the signaling of other inflammatory proteins. For example, a recent report demonstrated that peripheral administration of LPS or TNF-α in adult wild-type mice caused elevated levels of proinflammatory cytokines (including IL-1β) in brain and subsequent loss of dopaminergic neurons in the substantia nigra, suggestive of Parkinsonian-like pathogenesis (18). Thus, it will be important to evaluate the effects of immune mediators not only in terms of AD pathogenesis, but also for all neurodegenerative processes. Furthermore, the stage of AD pathogenesis may influence the ability of IL-1β overexpression to clear Aβ; thus, activating IL-1β in very old APP/PS1 mice, which the authors plan to investigate, should yield important new information and help to clarify the role of this important cytokine in AD. Finally, it will be interesting to determine the long-term effects of overexpressing IL-1β. Specific activation of immune cells or inflammatory pathways may have potential protective benefits for AD, but some caution should be exercised to avoid switching the intended effect to that of a deleterious immune response resulting in cellular damage. The IL-1β results reported here (5) provide much food for thought and will undoubtedly encourage much-needed future investigation into a positive role for inflammation in neurodegenerative diseases, including AD. The IL-1βXAT mouse model provides a valuable new tool with which to begin to dissect the spatial and temporal contribution of this important cytokine in AD.
Acknowledgments
The author wishes to thank David Cribbs (University of California, Irvine, California, USA) and Piet Eikelenboom (University of Amsterdam, Amsterdam, The Netherlands) for thoughtful discussions on the topic of inflammation in AD. Wolfgang Notsch is thanked for his assistance in designing Figure 1.
Footnotes
Nonstandard abbreviations used: Aβ, amyloid β; AD, Alzheimer disease; APP, amyloid precursor protein; APP/PS1+IL-1β mouse, mouse expressing the APP/PS1 and IL-1βXAT transgenes; FIV, feline immunodeficiency virus; Iba-1, ionized calcium-binding adaptor molecule 1; PS1, presenilin 1; XAT, excisional activation transgene.
Conflict of interest: The author received partial research funding from Elan Corporation and Wyeth Pharmaceuticals to study Aβ immunotherapy during the past 3 years. The author’s spouse is an employee and stockholder of Genzyme.
Citation for this article: J. Clin. Invest. 117:1483–1485 (2007). doi:10.1172/JCI32356.
See the related article beginning on page 1595.
References
- 1.Morgan D., Gordon M.N., Tan J., Wilcock D., Rojiani A.M. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer’s therapeutics. J. Neuropathol. Exp. Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 2.Eikelenboom P., et al. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 3.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe M. Shutting down Alzheimer’s. Sci. Am. 2006;294:72–79. doi: 10.1038/scientificamerican0506-72. [DOI] [PubMed] [Google Scholar]
- 5.Shaftel S.S., et al. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyss-Coray T., et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 7.Frenkel D., Maron R., Burt D.S., Weiner H.L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears β-amyloid in a mouse model of Alzheimer disease. J. Clin. Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim G.P., et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model of Alzheimer’s disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jantzen P.T., et al. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal antiinflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyss-Coray T., et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl. Acad. Sci. U. S. A. . 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seabrook T.J., Jiang L., Maier M., Lemere C.A. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- 12.El Khoury J., et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 13.Colangelo V., et al. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 14.Griffin W.S., et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. . 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das P., et al. Interleukin-1 receptor 1 knockout has no effect on amyloid deposition in Tg2576 mice and does not alter efficacy following Aβ immunotherapy. J. Neuroinflammation. 2006;3:17. doi: 10.1186/1742-2094-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Liu L., Barger S.W., Griffin W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng J.G., Zhu S.G., Jones R.A., Griffin W.S.T., Mrak R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 2000;163:388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin L., et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462.. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]