Abstract
The transfusion of lymphocytes, referred to as adoptive T cell therapy, is being tested for the treatment of cancer and chronic infections. Adoptive T cell therapy has the potential to enhance antitumor immunity, augment vaccine efficacy, and limit graft-versus-host disease. This form of personalized medicine is now in various early- and late-stage clinical trials. These trials are currently testing strategies to infuse tumor-infiltrating lymphocytes, CTLs, Th cells, and Tregs. Improved molecular biology techniques have also increased enthusiasm and feasibility for testing genetically engineered T cells. The current status of the field and prospects for clinical translation are reviewed herein.
Over the past 50 years, two fundamentally different strategies to stimulate antitumor immunity have been tested in humans: therapeutic vaccination and passive immunization. Passive immunization, herein referred to as adoptive T cell therapy, is the transfusion of autologous or allogeneic T cells into tumor-bearing hosts, i.e., patients. Evidence that T cells can help to control tumor growth has been provided by the analysis of tumor prevalence in immunodeficient mice and humans (1, 2). In the 1970s, Chester Southam and colleagues demonstrated that subcutaneous growth of human tumor autografts to patients bearing advanced cancers was inhibited by cotransfer of autologous leukocytes in about half of the patients (3). This suggested that leukocytes with a specific inhibitory effect on the implantation and growth of cancer cells were present in many patients with advanced cancer and could be used as potential candidates for adoptive immunotherapy. Furthermore, recent evidence indicates that tumor infiltration by human T cells is a powerful predictive biomarker of survival for ovarian and colorectal cancers (4, 5).
Therapeutic cancer vaccines are entering the realm of clinical medicine, but despite more than 60 years of research into this therapeutic approach (6), there are currently no FDA-approved adoptive T cell therapies for cancer. However, the recent explosion of knowledge in the fields of T cell and cancer biology has enabled new approaches that might bring adoptive T cell transfer to the routine practice of clinical medicine, with an impact similar to that of the advent of transfusion medicine, which was enabled by blood bank transfusion technology in the first half of the last century. The application of recent lessons from adoptive transfer in lymphodepleted hosts (7), the ability to overcome barriers presented by Tregs (8, 9), and the use of improved culture systems (10) have not yet been tested in randomized clinical trials. The intent of this review on the use adoptive T cell therapy for cancer in the clinic is to focus on issues facing the field, with an emphasis on therapy with CTLs, tumor-infiltrating lymphocytes (TILs), engineered T cells, and the use of adoptive T cell transfers to facilitate therapeutic cancer vaccines.
CTL therapy
At present, there is a plethora of suitable CTL targets for many tumors (11). Improved CTL cell culture technology (12) has permitted the first clinical tests of adoptive transfer of CTLs, and the approach seems to result in substantial activity in patients with melanoma; CTLs derived from PBLs were used to treat patients with refractory, metastatic melanoma, and 8 of the 20 patients had minor, mixed, or stable antitumor immune responses (13). Furthermore, the infusion of autologous melanoma-associated antigen recognized by T cells 1–specific (MART-1–specific) CD8+ T cells into a patient with metastatic melanoma resulted in T cell infiltration into both the skin and tumor tissue (14). The in vivo efficacy of the infused T cell population was indicated by the destruction of normal melanocytes and outgrowth of a MART-1–negative tumor, demonstrating the selection of a tumor variant with loss of MART-1 expression (14). These results were confirmed in an independent trial in which engraftment of the CTLs, as measured by an elevated frequency of circulating T cells able to bind tetramers loaded with MART-1 peptides, was detectable up to two weeks after T cell transfer in all patients, with a maximal frequency of 2% of the total CD8+ T cells (15). Despite this high level of engraftment in all patients, only 3 of 11 patients had clinical antitumor responses, and a selective loss of MART-1 expression in lymph node metastases in 2 of 2 evaluated patients was observed (15). Therefore, perhaps the most worrisome issue revealed with CTL transfers is the emergence of antigen escape variants, which seems to be more common in human tumors than in mouse syngeneic tumor models (16, 17). However, preliminary results have indicated that CTL transfers in patients with melanoma might have a vaccine-like effect, inducing epitope spreading, in that the antitumor response correlated with the detection of T cell clones with higher avidity for the tumor antigen and with a broader tumor antigen–specific repertoire than was detected before treatment (18). Therefore, it is possible that the problem of antigen escape variants can be addressed by enhancing the immune response to include a broad tumor antigen–specific T cell repertoire, either by increasing the efficiency of epitope spreading or by infusing CTL clones with multiple antigenic specificities.
TIL therapy
Adoptive transfer therapy with TILs requires the isolation of T cells from fresh patient biopsy specimens and the progressive selection of tumor-specific T cells ex vivo using high levels of IL-2 and various cell culture approaches (Figure 1). The adoptive transfer of these cells showed promise in preclinical models (19), but clinical experiences, with perhaps one exception (20), were almost uniformly disappointing (21–23). However, recent studies at the National Cancer Institute suggest that prior host conditioning with chemotherapy increases the response to adoptive immunotherapy with TILs (7, 24). When 13 patients with progressive metastatic melanoma were given cyclophosphamide and fludarabine, a drug regimen that is immunosuppressive but does not have anti-melanoma efficacy, 6 patients had partial responses as judged by Response Evaluation Criteria in Solid Tumors (RECIST; ref. 25), and 4 others had mixed responses, i.e., some of their tumors regressed but others remained (7). This approximately 50% objective response rate was confirmed in a subsequent report from the same group (24). Importantly, the TILs showed prolonged engraftment compared with TILs transfused to patients without prior treatment with these chemotherapeutics, and the levels of engraftment correlated with the clinical responses. Indeed, concomitant host immunosuppression seems to be important because only 34% of patients with melanoma who were treated with TIL administration and high-dose IL-2 and who received no prior chemotherapeutic conditioning therapy to induce lymphodepletion achieved objective clinical responses in trials previous to the incorporation of host lymphodepletion (21); most of the responses were transient, and the patients had limited persistence of the transferred cells in those trials. Adverse effects in the lymphodepletion trial included opportunistic infections and the frequent induction of vitiligo and uveitis, presumably due to autoimmunity. However, at this point, the results are difficult to interpret, as the ability to successfully generate TILs for therapy could be a predictive biomarker of a more favorable clinical outcome (4, 5, 26, 27). Therefore, in the absence of a randomized clinical trial it is not possible to determine how much lymphoablative chemotherapy, high-dose IL-2 administration, and TIL therapy contributed to the promising results in these recent trials (7, 24). If it is confirmed that lymphodepletion augments TIL efficacy, the results from recent trials indicate that induction of immunosuppression in the host improves the antitumor efficacy of adoptive TIL therapy. Pre-clinical models suggest that the concomitant transfer of autologous HSCs might have an additional effect in promoting the antitumor efficacy of adoptively transferred T cells (28). This would suggest that a combination of autologous HSC transplant approaches with adoptive therapy could have improved clinical results and may explain some reports of autologous graft-versus-host disease (GVHD) (29).
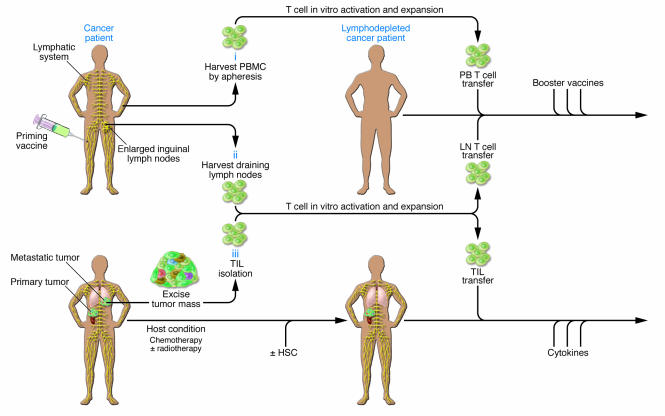
Figure 1. Schemes for adoptive transfer of autologous, vaccine-primed, in vitro–expanded T cells.
Patients are primed with tumor vaccine followed by lymphocyte harvest. Autologous T cells are harvested from peripheral blood (i) or draining lymph nodes (ii), undergo polyclonal in vitro activation and expansion, and are reinfused after lymphodepleting chemotherapy. Antigen-specific immune function is measured after the administration of booster vaccines. (iii) TILs can be isolated from resected surgical specimens and expanded in vitro for adoptive transfer after lymphodepleting chemotherapy. Most adoptive transfer therapy approaches using TILs have involved the use of IL-2 infusion following T cell transfer in order to select tumor-specific T cells.
Technical issues with producing tumor-specific T cells currently present a formidable barrier to conducting randomized clinical trials using TILs. Only 30%–40% of biopsy specimens yield satisfactory T cell populations, and the process is labor and time intensive, requiring about 6 weeks to produce the T cells for infusion (30). Therefore, randomized trials based on rigorous intent-to-treat analysis design (in which all data from all patients are included in the data analysis and any patients who are discontinued or otherwise nonevaluable are considered to be treatment failures) cannot be performed using currently available tissue culture technologies, and the trials reported to date have been performed based on an ad hoc, as-treated analysis plan. Furthermore, nearly all clinical experience with TILs has been with patients with melanoma because of the ready surgical availability of tumor biopsy tissue. However, should technical limitations of current tissue culture approaches be overcome, the recent studies indicating that the presence of TILs correlated positively with survival in ovarian and colorectal cancer (4, 5) could extend the impact of this promising therapeutic approach to other commonly encountered epithelial cancers.
Combination approaches using vaccines and adoptive T cell transfer
Due to the limited time window and practical constraints imposed by large tumor masses (because immediate tumor regression is desired), therapy is superior to therapeutic vaccination as a single therapeutic modality (31, 32), and therefore, as a corollary, the strategy of therapeutic tumor vaccination of cancer patients is likely to succeed mainly in the setting of minimal residual disease (33). In mice, adoptive T cell therapy enhances the effects of therapeutic vaccines (34, 35), and this combined approach in the setting of lymphopenia results in a further enhancement of tumor immunity compared with combined treatment in lymphoreplete hosts (36, 37). In humans with myeloma, idiotype vaccination of sibling donors with the unique tumor-specific Ig produced by the patient’s myeloma cells followed by adoptive transfer in the setting of allogeneic stem cell transplantation can result in the induction of potent antitumor immunity (38). However, although theoretically attractive, there is not yet extensive data in humans to demonstrate the efficacy of a combined vaccine and adoptive T cell transfer approach in the autologous setting.
Two phase I clinical trials using autologous activated T cells transplanted for hematologic malignancies in patients have been reported. In the first trial (39), patients with relapsed or chemotherapy refractory non-Hodgkin lymphoma were given a CD34+ HSC transplant followed by infusion of autologous T cells expanded ex vivo with CD3- and CD28-specific antibodies (40). Infusion of the autologous costimulated T cells resulted in a rapid reconstitution of lymphocyte numbers. Importantly, the expanded cells were functionally superior to those obtained directly from the patients, as determined by their ability to produce IFN-γ when stimulated with tumor cells in vitro. In a second randomized trial, the feasibility was tested of pre-transplant immunization and adoptive transfer of vaccine-primed T cells in the setting of autologous transplantation for multiple myeloma (41). Patients were vaccinated with Prevnar, the heptavalent pneumococcal conjugate vaccine (PCV), and two weeks later T cells were harvested and expanded in vitro using CD3- and CD28-specific antibodies. Patients received a standard autologous peripheral blood HSC transplant after melphalan conditioning, the ex vivo–expanded autologous T cells either 14 days or 100 days after transplant, and two doses of PCV beginning one month after transplant. Prompt T cell recovery was observed in patients that received ex vivo–expanded autologous T cells 14 days after the HSC transplant, whereas those that received the T cells 100 days after the transplant remained substantially lymphopenic. Early adoptive transfer of PCV-primed T cells resulted in the induction of potent immunity, as only those individuals who received PCV-primed T cells soon after the transplant developed and maintained protective levels of pneumococcal-specific antibodies as well as vaccine-specific CD4+ T cell responses. These data demonstrated that a combination of vaccine and adoptive T cell therapy, consisting of a single pre-transplant vaccine and an early post-transplant infusion of in vivo–antigen-primed, ex vivo–costimulated autologous T cells followed by booster immunizations improved the severe immunodeficiency associated with high-dose chemotherapy and led to clinically relevant immunity in adults within a month of HSC transplantation. This pilot study provides a useful foundation for the design of future strategies using combinations of tumor vaccines and vaccine-primed T cells in lymphodepleted patients with various malignancies (Figure 1).
Shu and Chang have developed an alternative vaccine adoptive transfer approach that could circumvent many of the limitations posed by adoptive transfer using TILs. They and others have shown in mice that tumor-draining lymph nodes harbor T cells that are not able to mediate tumor rejection in adoptive transfer experiments (42, 43). By contrast, if the draining lymph node cells are activated in vitro by various culture approaches, the cells are able to mediate tumor rejection after adoptive transfer (44). In a further study (45), T cells were isolated from vaccine-primed lymph nodes obtained from patients with melanoma, renal cell carcinoma, and head and neck cancer. In the absence of APCs, activation with CD3- and CD28-specific antibodies greatly enhanced subsequent T cell expansion in response to IL-2 compared with activation with CD3-specific antibody alone (45). Based on these and other preclinical data, Chang and coworkers carried out a phase I clinical trial in patients with either advanced melanoma or renal cell carcinoma (46). Patients were vaccinated with irradiated autologous tumor cells and Mycobacterium bovis bacillus Calmette-Guérin (BCG). The draining lymph nodes were harvested 7–10 days later and the vaccine-primed T cells cultured and infused (Figure 1). Among the 11 patients with melanoma, 1 had a partial tumor regression, and among the 12 patients with renal cell carcinoma, there were 2 complete and 2 partial tumor regressions (46), suggesting that there might be some clinical benefit to this adoptive transfer approach to boost the clinical efficacy of therapeutic cancer vaccines.
Engineered T cells
Genetic modification of T cells to engineer improved antitumor effects and enhanced immune reconstitution of immunosuppressed patients is an attractive strategy in many settings (47). In patients with congenital and acquired immunodeficiency, genetically modified T cells have been shown to persist for years in humans following adoptive transfer (48, 49), which indicates that the general approach is feasible. A potential safety concern when infusing individuals with engineered T cells is one that arose with genetically engineered HSCs (50), when viral insertional mutagenesis was shown to cause cellular transformation. Although there is little clinical experience with engineered T cells for cancer therapy, it is notable that clinical trials to date using cells engineered to express suicide molecules have indicated that the approach is safe.
T cells engineered to express suicide molecules.
Severe and potentially lethal GVHD represents a frequent complication of allogeneic immunotherapy and donor lymphocyte infusion (DLI). The promising results with DLI have created increased interest in developing T cells with an inducible suicide phenotype (Figure 2). Expression of herpes simplex virus thymidine kinase (HSV-TK) in T cells provides a means of ablating transduced T cells in vivo by the administration of acyclovir or ganciclovir (51). Using this strategy, Bordignon and colleagues infused allogeneic donor lymphocytes engineered to express HSV-TK into 8 patients with refractory hematologic malignancies who had suffered complications such as cancer relapse or virus-induced lymphomas after receiving allogeneic bone marrow transplants from the donor of the allogeneic lymphocytes (52). The lymphocytes survived for up to a year, and complete or partial tumor remission in five of the eight patients was achieved. Tumor regression coincided with onset of GVHD, and in most cases, GVHD was abrogated when ganciclovir was administered. A recent phase II clinical trial has confirmed and extended these results to further demonstrate the safety and feasibility of adoptive transfer of suicide gene–transduced donor T cells (53). Previously, transplantation of haploidentical HSCs had only been possible with T cell depletion of the allogeneic stem cell graft to prevent GVHD, but this resulted in profound immunodeficiency following transplant. The HSV-TK approach seems to promote immune reconstitution and preserve the antitumor effects of the adoptively transferred T cells in immunosuppressed recipients. It is possible that the first form of adoptive therapy with engineered T cells to enter clinical practice will be the use of allogeneic T cells engineered to have a conditional suicide switch, as a phase III clinical trial is planned to test this approach in the setting of haploidentical HSC transplantation.
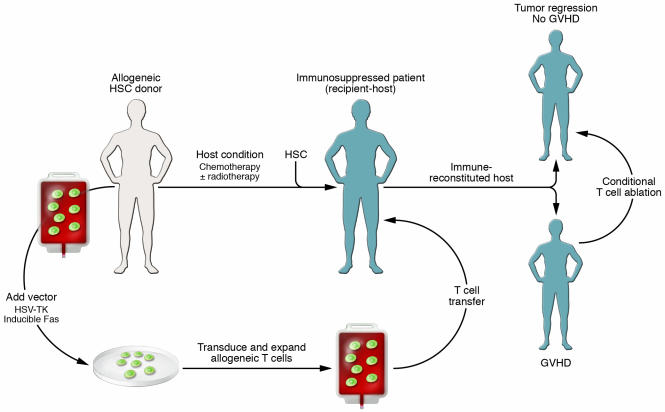
Figure 2. Suicide T cell therapy using adoptively transferred T cells.
T cells can be engineered to express conditional suicide switches so that the T cells die when a drug is administered that activates the switch and causes apoptosis. Suicide constructs have been incorporated into allogeneic T cells that can be ablated in the event of GVHD and into autologous T cells that can be ablated in the event of toxicity or uncontrolled T cell proliferation.
The principal concern with the HSV-TK approach has been that it would generate potent HSV-TK–specific immune responses, thereby inducing elimination of the adoptively transferred T cells independently of ganciclovir administration. For example, others have found that humans efficiently reject cells engineered to express HSV-TK or similar constructs (54, 55). Therefore, under conditions in which the host is not as profoundly immunosuppressed, such as in the case of haploidentical transplantation, HSV-TK might confer immunogenicity to the transfused cells, leading to their impaired survival and the inability to retreat a patient with a DLI of cells engineered to express HSV-TK should the tumor recur. Future development of vectors that encode less immunogenic proteins but are able to confer even higher ganciclovir sensitivity to transduced human T cells is required to extend this approach to immunocompetent hosts. Recently, investigators have developed suicide systems comprised of fusion proteins containing a human FAS or caspase death domain and a modified FK506-binding protein (FKBP) (56, 57). These approaches have the advantage that the suicide switches are expected to be nonimmunogenic because they are based on endogenous proteins. T cells expressing these modified chimeric proteins are induced to undergo apoptosis when exposed to a drug that dimerizes the modified FKBP (Figure 2).
T cells engineered to express tumor antigen–specific receptors.
A principal limitation of adoptive T cell therapy for some tumors is that the tumors are poorly antigenic; therefore, neither T cells with high avidity for tumor-specific antigens, nor T cells with the desired specificity remain in the patient following chemotherapy. Two strategies to overcome this limitation are now being tested in the clinic (Figure 3). One approach has been to endow T cells with novel receptors by introduction of “T bodies,” chimeric receptors that have antibody-based external receptor structures and cytosolic domains that encode signal transduction modules of the T cell receptor (58). These constructs can function to retarget T cells in vitro in an MHC-unrestricted manner to attack the tumor while retaining MHC-restricted specificity for the endogenous TCR. Three pilot clinical trials have recently been reported. A trial that tested T cells expressing a T body receptor specific for a folate-binding protein that is present on ovarian carcinoma cells indicated that the approach was safe, but poor expression and persistence of the transgene encoding the T body receptor were observed in vivo (59). Similarly, a pilot test in children with neuroblastoma treated with autologous T cells retargeted for a tumor-associated adhesion molecule has indicated that the approach is safe but was limited by poor persistence of the T cells (60). Lamers and colleagues recently tested T cells expressing a T body receptor specific for carbonic anhydrase IX, an antigen present on the surface of clear cell renal cell carcinoma (61). They observed an unexpected serious hepatic toxicity in several patients within a week of T cell infusion that seemed to be due to carbonic anhydrase IX expression in the biliary tract. If confirmed, this would indicate that engineered T cells can traffic to and exert effector function at sites of antigen expression in vivo. Furthermore, this study indicates that the targets of chimeric antigen receptors must be carefully chosen to avoid unwanted adverse effects, or that additional safety features, such as suicide switches, need to be incorporated into the vectors driving the expression of the chimeric receptor. In several of the patients in the studies described above, the engineered cells persisted for several days to weeks before elimination by host immune responses (59–61), indicating that a technical challenge for this approach is to prevent a host immune response from eliminating the adoptively transferred cells. The other major issues with the approach currently involve improving receptor design by optimizing the ligand-binding domain and by trying to incorporate costimulatory signaling domains into the signaling module (62). The former issue is important because the avidity of the ligand-binding domain must be tuned to afford specificity for the tumor cells and yet permit disengagement from the target so that the redirected T cells can be “serial killers.” The later issue is important to ensure long-term survival of the T cells so that the proper costimulatory signals can be delivered upon tumor recognition, thereby avoiding the induction of anergy or apoptosis (63) and by potentially increasing the resistance of the T cells to the immunotoxic effects of the tumor microenvironment (64).
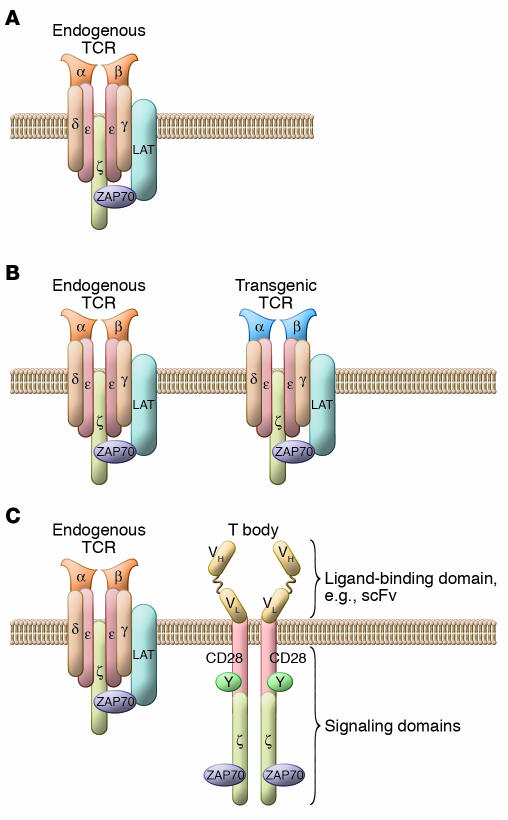
Figure 3. T cells can be engineered to have retargeted specificity for tumors.
(A) Endogenous T cells express a single heterodimeric TCR. (B) Bispecific T cells are created by the introduction of genes that encode proteins that recognize antigens expressed by target tumor cells. These genes can encode natural TCRs that function in the same MHC-restricted manner as endogenous TCRs but have tumor antigen specificity. (C) Alternatively, these genes can encode chimeric tumor antigen–specific receptors, or T bodies, that target surface antigens in an MHC-independent fashion. T bodies express an extracellular ligand generally derived from an antibody and intracellular signaling modules derived from T cell–signaling proteins. LAT, linker for activation of T cells; ScFv, single chain variable fragment; ZAP70, ζ-chain–associated protein kinase 70 kDa.
T cells are also being transduced to express natural αβTCR heterodimers of known specificity and avidity for tumor antigens (65). In the first clinical trial using this approach, T cells were engineered to express a TCR specific for glycoprotein 100 (gp100), and lymphodepleted patients with melanoma were given a single infusion of these engineered T cells followed by an infusion of IL-2 (66). A concern with this approach has been that it might generate additional, novel receptor specificities by pairing of the transgenes with the endogenous TCR chains. It is encouraging that no toxicity was observed in the pilot trial, and promising persistence of the engineered T cells was observed in some of the patients. However, one issue that arose was low cell-surface levels of expression of the gp100-specific TCR, which would be expected to lower the avidity of the TCR and therefore minimize effector functions. Another general limitation of this approach for humans is that each TCR is specific for a given peptide-MHC complex, such that each vector would only be useful for patients that shared both MHC alleles and tumor antigens.
T cells engineered for enhanced survival.
A limitation to adoptive transfer of CTLs is that they have short-term persistence in the host in the absence of antigen-specific Th cells and/or cytokine infusions. Greenberg and coworkers have transduced human CTLs with chimeric GM-CSF–IL-2 receptors that deliver an IL-2 signal when they bind GM-CSF (67). Stimulation of the CTLs with antigen caused GM-CSF secretion and resulted in an autocrine growth loop such that the CTL clones proliferated in the absence of exogenous cytokines. This type of genetic modification has the potential to increase the circulating half-life of the CTLs and, by extension, the efficacy of these ex vivo–expanded cells. A related strategy to rejuvenate T cell function is to engineer T cells to ectopically express CD28 (68) or the catalytic subunit of telomerase (69).
The future of adoptive therapy with engineered T cells.
The field of adoptive therapy with engineered T cells is on the cusp of substantial clinical advances that are now possible because of improved cell culture and gene transfer methods. Unlike HSCs, currently available retroviral vectors provide high-level expression of transgenes in T cells in vitro, although silencing of expression might be a challenge for long-term in vivo therapies (70). The advent of lentiviral vectors has greatly increased the efficiency of human T cell engineering, and a recent pilot study with lentiviral engineered T cells that expressed an anti-sense HIV vector showed promise in patients infected with HIV (71). As mentioned above, insertional mutagenesis is a safety concern with any integrating viral vector. It is reassuring that the natural history of HIV does not include an increased incidence of T cell leukemia; this provides empirical data that lentiviral vectors might be safer in this respect than oncoretroviral vectors. Furthermore, side-by-side tests in preclinical models indicate that lentiviral vectors are less prone to insertional mutagenesis (72). Nevertheless, long-term observational studies with large patient safety data sets are required to determine the ultimate safety of this approach. Finally, a primary issue that could limit the ultimate efficacy of the approach is the immunogenicity of the proteins that the T cells are engineered to express; this is likely to be a larger problem in humans than in mice because activated human T cells, unlike mouse T cells, express MHC class II molecules and have been shown to function as effective APCs (73).
Strategies to augment the efficacy of adoptively transferred T cells
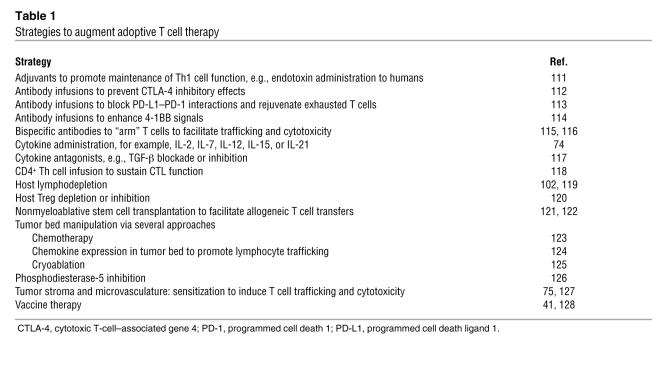
There are a number of strategies that might augment the function of adoptively transferred T cells (Table 1). The efficacy of adoptive transfer is enhanced by other immunotherapies such as cytokine administration (74), and in some circumstances, by standard cytotoxic chemotherapy and radiation (75, 76). Recent studies indicate that the choice of chemotherapy might be more important than was previously realized, as some cytotoxic agents render tumor cells more immunogenic than others, independent of the fractional killing effect of the drug (77, 78). Genetic engineering of T cells has the potential to augment function through various cell autonomous mechanisms, as discussed above. In addition, T cells can be engineered for resistance to cell extrinsic forms of immunosuppression such as those mediated by TGF-β and Tregs (64, 79). Therefore, as with other forms of immunotherapy, it is probable that the ultimate clinical application of adoptive T cell transfer will employ combinatorial approaches (80).
Table 1 .
Strategies to augment adoptive T cell therapy
Clinical trials
A premise of this Review is that clinical trials of adoptive T cell transfer based on a sufficient understanding of lymphocyte and cancer biology have only begun in recent years. Nevertheless, lessons can be learned from previous trials that failed to achieve the expected clinical efficacy. In addition, issues related to the clinical translation of adoptive T cell transfer therapy are discussed below, with an emphasis on dose and scheduling issues, potential toxicities, and the optimal antigens to target with adoptively transferred T cells.
Status.
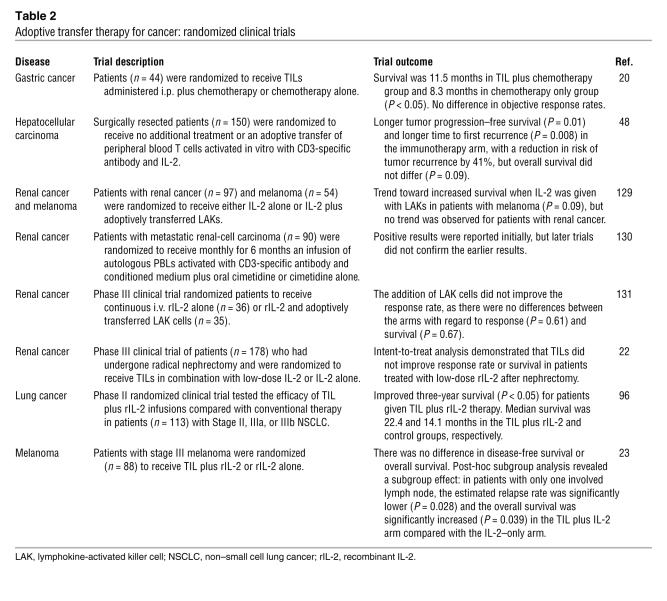
Pilot clinical trials of adoptive T cell immunotherapy were initiated in cancer soon after the discovery of IL-2, which enabled the large scale culture of T cells for the first time (81). However, until recently, the clinical trials have been carried out with populations of cells that we now know were rendered tolerant or “anergic”, senescent, or immunogenic. The major criticism of the field has been that, until recently, no randomized clinical trials had demonstrated that adoptive T cell transfer approaches were efficacious (Table 2). The adoptive transfer of EBV-specific T cell lines and CTLs for the therapy of EBV-induced lymphomas is perhaps the best demonstration of clinically efficacious adoptive T cell therapy (82, 83). However, the EBV-induced lymphomas that occur in immunosuppressed patients are a “disappearing disease,” as advances in treatment to use a CD20-specific antibody (Rituximab) have drastically reduced the incidence of this once not uncommon disorder. Therefore, in the case of EBV-associated malignancies, a randomized efficacy trial is not likely to occur. To date, there has only been one randomized clinical trial that had a positive outcome; a rigorous intent-to-treat analysis of adoptive transfer trial in cancer has been in the adjuvant setting for hepatocellular carcinoma following surgical resection of the primary tumor (84). In that study, autologous peripheral blood T cells were cultured with CD3-specific antibody and IL-2, and the risk of cancer recurrence was reduced by 41% in the group treated with surgery and a T cell infusion compared with the group treated with surgery only. However, this trial remains unconfirmed, and the mechanism of the antitumor effect remains unknown. It is critical to learn whether a specific antitumor effect or an antiviral response directed to HCV, the agent often implicated in the pathogenesis of hepatocellular carcinoma (85), was involved in the protective effect. It is also conceivable that other effects, such as a reduction in the suppressive effect of Tregs, could have occurred in this trial as well. These are important lessons to learn, as hepatocellular carcinoma is the third leading cause of cancer-related deaths worldwide.
Table 2 .
Adoptive transfer therapy for cancer: randomized clinical trials
Dose and scheduling issues.
Information on the dose and schedule dependence of adoptively transferred cells is widely scattered in the literature, and from this literature one concludes that there is no standardized dosage system. There is, however, evidence from animal models (in nonlymphopenic hosts) suggesting that multiple doses of adoptively transferred T cells are superior to a single infusion of T cells (86). Doses of adoptively transferred cells are usually reported as the total number of viable cells administered or as the total number of viable cells administered per kilogram of body weight or per square meter of body surface area. However, total endogenous lymphocyte numbers do not correlate well with body surface area but rather display a strong inverse correlation with age. Other variables add to the complexity, particularly the fact that, in the case of T cells or other adoptively transferred cells with high replicative potential, the infused dose might not relate well to the steady-state number of cells that engraft and persist. Therefore, dose considerations are more complex than in other areas of transfusion medicine, where, for example, the maximal level of transfused red cells or platelets occurs immediately following infusion. In our studies of adoptively transferred autologous CD4+ T cells, we often find that the number of cells in the host peaks two weeks after infusion of the cells (87). This is because the engraftment potential and the replicative potential of the infused cells depends on complex host variables such as the number of niches available in the host for engraftment, and the antigenic stimulus for clonal expansion or deletion. In most rodent tumor models, T cell proliferation in the host after transfer is obligatory for therapeutic efficacy (reviewed in ref. 88), and with rare exceptions (89), this is presumed to also be required in humans.
Cytokines given to the host can also have a major impact on the persistence of adoptively transferred T cells. Others have found that the persistence of adoptively transferred human CD8+ T cells is enhanced by coadministration of IL-2 (13). However, we have found that when autologous human CD4+ T and CD8+ T cells are given in combination, persistence is not increased by concomitant IL-2 therapy (49). Finally, recent studies show that IL-2 can induce the proliferation and maintenance of effector CD8+ T cells but might actually deplete memory T cells and increase the number of Tregs (90). By contrast, IL-15 and IL-7 seem to select for the persistence of memory CD8+ T cells and might decrease the ratio of Tregs to effector T cells (91).
Striking schedule-dependent increases in efficacy and the frequency of adverse effects from adoptively transferred cells have been reported when T cell infusions are given to lymphopenic hosts (7). Many studies in rodent tumor models show that the coadministration of cytotoxic therapy can enhance the effects of adoptively transferred cells (92). Cyclophosphamide and/or fludarabine are generally administered to the host several days before the adoptively transferred T cells (7, 88). The drugs have multiple effects that seem to promote the antitumor effects of the adoptively transferred T cells. There is evidence for numerous effects, including killing of host Tregs that suppress antitumor immune responses; creating “space” in the host so that the adoptively transferred T cells can engraft (93); and perhaps enhancing cross-priming of tumor antigens. Curti and colleagues (94), have studied the optimal time to harvest autologous CD4+ T cells in relation to the timing of cyclophosphamide administration in patients with advanced cancers. T cells were harvested at steady state or either when on the decline or recovery from the cyclophosphamide-induced leukopenia, and Curti et al. found the greatest in vivo CD4+ T cell expansion following infusion when cells were harvested as patients entered the cyclophosphamide-induced nadir (94). In a study of patients with stage III non–small cell lung cancer, investigators tested the sequence of adoptive therapy with autologous TIL and IL-2 followed by standard chemotherapy and radiotherapy, and perhaps not surprisingly, they found that immunotherapy followed by chemotherapy was not effective (95); the reverse schedule of therapy was not tested as a concurrent comparison in this trial, however previous randomized trials from this group had demonstrated clinical activity when chemotherapy was followed by immunotherapy (96).
Toxicity issues.
Many types of adverse events have been reported following infusion of human autologous or allogeneic lymphocytes. The toxicities can be classified as those that result from extrinsic factors present in the culture process, those resulting from accompanying cytokines that can be co-infused with the cells, and those that result from the cells themselves. The spectrum of the third form of adverse effects is still being defined and for the moment seems to be related to whether the cell product is genetically engineered. For cell products that have not been genetically engineered, the adverse effects are limited and are similar to those observed with therapeutic vaccines. Cytokine release syndrome, retinitis, iritis, hepatitis, autoimmune thyroiditis with hypothyroidism, and vitiligo occur following autologous T cell infusions (7, 14, 61, 97). Respiratory obstruction has been reported following CTL infusion for EBV-related lymphomas (82). This is probably due to a T cell–induced inflammatory response that results in tumor edema and necrosis. Effector functions of infused T cells can be expected to include tissue damage similar to that encountered in T cell–mediated autoimmune diseases. In the case of allogeneic lymphocyte infusions, GVHD and bone marrow aplasia can occur (98). Theoretic toxicities associated with T cell transfer also include leukemia or lymphoma if transformation is induced consequent to the in vitro culture process. However, in human trials involving genetically modified T cells, no cases of malignant transformation of the infused T cells have been reported to date.
Finally, dose- and schedule-dependent effects have been observed with allogeneic T cell infusions vis-à-vis the induction of GVHD. Early studies showed that the infusion of donor T cells soon after a myeloablative transplant conditioning regimen resulted in the marked augmentation of acute GVHD (99). It has been well established by the work of O’Reilly and colleagues that the initial dose of infused T cells in the setting of allogeneic bone marrow transplantation has a major effect on the incidence and severity of acute GVHD (98). However, it has only been recently appreciated that donor T cells can be infused with relative freedom from acute GVHD in the setting of nonmyeloablative stem cell transplantation (100). Studies show that, in the steady-state setting of relapsed chronic myelogenous leukemia following allogeneic HSC transplantation, infusions of resting donor T cells result in a decreased incidence of acute GVHD when given by dose fractionation, starting with low doses of donor cells and escalating subsequent doses as required (101). Some of these effects might be related to recent findings in mice that effector CD8+ T cell function and presumably toxicity are related to concomitant HSC infusion (28).
Tregs.
Cancer patients have increased numbers and function of CD4+CD25+ Tregs at the tumor site (8). The in vivo depletion of Tregs enhances the antitumor effects of adoptively transferred effector T cells (102). On the other hand, preclinical models show that the adoptive transfer of Tregs was able to prevent GVHD while preserving graft versus tumor activity (103). Recently, we and others have developed ex vivo culture conditions that should permit pilot trials of Treg adoptive immunotherapy for the prevention or therapy of GVHD (104, 105).
Targeting issues: public versus private antigen controversy.
There is controversy in the choice of antigen to target with adoptively transferred T cells. For the past several decades, shared (also known as “public”) tumor-associated antigens have been the favored target of various immunotherapy strategies. This approach has been based largely on melanoma and has been led by a study of the CTLs obtained from a patient with melanoma (106). Most of the antigens targeted by T cells obtained from patients with regressing melanoma had expression that was shared between tumor cells and their normal cell counterparts. Implications from these shared tumor–associated antigens were that, in order to achieve tumor eradication it was necessary to expect tissue-specific toxicity, such as vitiligo in the case of melanoma and prostatitis in the case of prostate cancer. Therefore, the concept of “dispensable tissues” arose (107), meaning that in the case of some tumors, damage or destruction of normal tissue would be an accepted and expected potential toxicity. Because expression of these antigens was also shared between different individuals, the preparation of patient-independent vaccine preparations would be possible. In theory however, patient-specific (also known as “private”) tumor antigens that arise from mutations could also serve as a source of tumor-specific targets. Strategies to target patient- and tumor-specific mutations have been proposed but have not received much attention in the field (108, 109). This situation is likely to change given the striking finding that common tumors such as breast and colon cancer have, on average, about 90 mutations per tumor that generate amino acid substitutions (110), a figure much higher than was previously thought. These findings have major implications for cancer immunotherapy, as a strategy that is directed against patient- and tumor-specific antigens is likely to have fewer off target effects. In addition, it might be possible to generate T cells with much higher avidity for the tumor target, since the TCR repertoire to these putative tumor-specific antigens is not expected to have been subject to editing by thymic tolerance mechanisms. By contrast, strategies targeting shared tumor-associated antigens are hindered by T cell responses against self antigens that are generally of low avidity and susceptible to immunologic tolerance.
Conclusions
Adoptive T cell therapy is the ultimate challenge to implementation of personalized medicine. To be commercially viable, adoptive T cell therapy has to be clinically effective, scalable, reproducibly manufactured, and appropriately priced and marketed. Will therapy be delivered using a blood banking model or by centralized manufacturing plants? It is probable that engineered T cell therapies will require stringent manufacturing controls that favor centralized manufacturing plants, whereas some forms of manufacturing for natural T cell therapies could be carried out at tertiary care medical centers. The anticipated approval of a therapeutic cancer vaccine for prostate cancer based on autologous DCs is on the near horizon, which suggests that many of these challenges can be addressed. However the major challenge facing the field at present is to conduct randomized clinical trials demonstrating sufficient clinical benefit to justify the logistics and expense of customized cellular therapies.
Acknowledgments
The author thanks Bruce Levine, James Riley, Richard Carroll, Robert Vonderheide, and George Coukos for helpful discussions and Coral Haas for administrative help. The author is grateful for support of these studies by NIH grants 5R01CA105216, 1R01CA104679, and 5P50CA083638, the Leukemia and Lymphoma Society, and the Alliance for Cancer Gene Therapy. The author is grateful for advice from many colleagues and collaborators over the years and, due to constraints on references, apologizes for the inability to cite all relevant references.
Footnotes
Nonstandard abbreviations used: DLI, donor lymphocyte infusion; GVHD, graft-versus-host disease; HSV-TK, herpes simplex virus thymidine kinase; MART-1, melanoma-associated antigen recognized by T cells 1; TIL, tumor-infiltrating lymphocyte.
Conflict of interest: C.H. June receives income from BD Biosciences, Cell Genesys, CellDex Therapeutics, Maxcyte, and AVEO Pharmaceuticals Inc. and research support from BD Biosciences, GlaxoSmithKline, and Sangamo BioSciences. He also has patents and patent applications in the fields of adoptive T cell therapy and immunosuppression.
Citation for this article: J. Clin. Invest. 117:1466–1476 (2007). doi:10.1172/JCI32446.
References
- 1.Shankaran V., et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 2.Goedert J.J. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin. Oncol. 2000;27:390–401. [PubMed] [Google Scholar]
- 3.Southam C.M., Brunschwig A., Levin A.G., Dizon Q.S. Effect of leukocytes on transplantability of human cancer. Cancer. 1966;19:1743–1753. doi: 10.1002/1097-0142(196611)19:11<1743::aid-cncr2820191143>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. . N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Galon J., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Mitchison N.A. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J. Exp. Med. 1955;102:157–177. doi: 10.1084/jem.102.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley M.E., et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo E.Y., et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non small cell lung cancer and late-stage ovarian cancer. . Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 9.Peng L., et al. Tumor-induced L-selectin high suppressor T cells mediate potent effector T cell blockade and cause failure of otherwise curative adoptive immunotherapy. J. Immunol. 2002;169:4811–4821. doi: 10.4049/jimmunol.169.9.4811. [DOI] [PubMed] [Google Scholar]
- 10.June C.H. Principles of adoptive T cell cancer therapy. J. Clin. Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 12.Jotereau F., et al. High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J. Immunother. 1991;10:405–411. doi: 10.1097/00002371-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Yee C., et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee C., et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J. Exp. Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackensen A., et al. Phase I study of adoptive T-cell therapy using antigen-specific CD8(+) T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 16.Marincola F.M., Jaffee E.M., Hicklin D.J., Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 17.Norell H., et al. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–6394. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 18.Vignard V., et al. Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J. Immunol. 2005;175:4797–4805. doi: 10.4049/jimmunol.175.7.4797. [DOI] [PubMed] [Google Scholar]
- 19.Alexander R.B., Rosenberg S.A. Long-term survival of adoptively transferred tumor-infiltrating lymphocytes in mice. J. Immunol. 1990;145:1615–1620. [PubMed] [Google Scholar]
- 20.Kono K., et al. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin. Cancer Res. 2002;8:1767–1771. [PubMed] [Google Scholar]
- 21.Rosenberg S.A., et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 22.Figlin R.A., et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J. Clin. Oncol. 1999;17:2521–2529. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 23.Dreno B., et al. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol. Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley M.E., et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. . J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therasse P., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Sato E., et al. Intraepithelial CD8(+) tumor-infiltrating lymphocytes and a high CD8(+)/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pages F., et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. . N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 28.Wrzesinski C., et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J. Clin. Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmberg L., et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biol. Blood Marrow Transplant. 2006;12:226–234. doi: 10.1016/j.bbmt.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Dudley M.E., Wunderlich J.R., Shelton T.E., Even J., Rosenberg S.A. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romieu R., et al. Passive but not active CD8+ T cell-based immunotherapy interferes with liver tumor progression in a transgenic mouse model. . J. Immunol. 1998;161:5133–5137. [PubMed] [Google Scholar]
- 32.Hanson H.L., et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teshima T., Liu C., Lowler K.P., Dranoff G., Ferrara J.L.M. Donor leukocyte infusion from immunized donors increases tumor vaccine efficacy after allogeneic bone marrow transplantation. . Cancer Res. 2002;62:796–800. [PubMed] [Google Scholar]
- 35.Parviz M., et al. Successful adoptive immunotherapy with vaccine-sensitized T cells, despite no effect with vaccination alone in a weakly immunogenic tumor model. Cancer Immunol. Immunother. 2003;52:739–750. doi: 10.1007/s00262-003-0405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori S., Kocak U., Shaw J.L., Mullen C.A. Augmentation of post transplant immunity: antigen encounter at the time of hematopoietic stem cell transplantation enhances antigen-specific donor T-cell responses in the post transplant repertoire. Bone Marrow Transplant. 2005;35:793–801. doi: 10.1038/sj.bmt.1704883. [DOI] [PubMed] [Google Scholar]
- 37.Wang L.X., et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neelapu S.S., et al. Tumor antigen immunization of sibling stem cell transplant donors in multiple myeloma. Bone Marrow Transplant. 2005;36:315–323. doi: 10.1038/sj.bmt.1705057. [DOI] [PubMed] [Google Scholar]
- 39.Laport G.G., et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 40.Levine B.L., et al. Effects of CD28 costimulation on long term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 41.Rapoport A., et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat. Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 42.Tuttle T.M., et al. Activation and growth of murine tumor-specific T-cells which have in vivo activity with bryostatin 1. Cancer Res. 1992;52:548–553. [PubMed] [Google Scholar]
- 43.Shu S.Y., Chou T., Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. . J. Immunol. 1989;143:740–748. [PubMed] [Google Scholar]
- 44.Yoshizawa H., Chang A.E., Shu S. Specific adoptive immunotherapy mediated by tumor-draining lymph node cells sequentially activated with anti-CD3 and IL-2. J. Immunol. 1991;147:729–737. [PubMed] [Google Scholar]
- 45.Li Q., Furman S.A., Bradford C.R., Chang A.E. Expanded tumor-reactive CD4+ T-cell responses to human cancers induced by secondary anti-CD3/anti-CD28 activation. Clin. Cancer Res. 1999;5:461–469. [PubMed] [Google Scholar]
- 46.Chang A.E., et al. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J. Clin. Oncol. 1997;15:796–807. doi: 10.1200/JCO.1997.15.2.796. [DOI] [PubMed] [Google Scholar]
- 47.Ho W.Y., Blattman J.N., Dossett M.L., Yee C., Greenberg P.D. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 48.Blaese R.M., et al. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 49.Mitsuyasu R.T., et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ Gene-modified autologous CD4+ and CD8+ T cells in HIV-infected subjects. . Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- 50.Hacein-Bey-Abina S., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 51.Helene M., Lake-Bullock V., Bryson J.S., Jennings C.D., Kaplan A.M. Inhibition of graft-versus-host disease. Use of a T cell-controlled suicide gene. . J. Immunol. 1997;158:5079–5082. [PubMed] [Google Scholar]
- 52.Bonini C., et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 53.Ciceri F., et al. Anti-tumor effects of HSV-TK engineered donor lymphocytes after allogeneic stem cell transplantation. Blood. 2007 doi: 10.1182/blood-2006-05-023416. In press. [DOI] [PubMed] [Google Scholar]
- 54.Riddell S.R., et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 55.Berger C., Flowers M.E., Warren E.H., Riddell S.R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clackson T., et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Straathof K.C., Spencer D.M., Sutton R.E., Rooney C.M. Suicide genes as safety switches in T lymphocytes. Cytotherapy. 2003;5:227–230. doi: 10.1080/14653240310001497. [DOI] [PubMed] [Google Scholar]
- 58.Eshhar Z., Waks T., Bendavid A., Schindler D.G. Functional expression of chimeric receptor genes in human T cells. J. Immunol. Methods. 2001;248:67–76. doi: 10.1016/s0022-1759(00)00343-4. [DOI] [PubMed] [Google Scholar]
- 59.Kershaw M.H., et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J.R., et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 61.Lamers C.H., et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 62.Sadelain M., Riviere I., Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 63.Riley J.L., June C.H. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 64.Loskog A., et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 65.Schumacher T.N. T-cell-receptor gene therapy. . Nat. Rev. Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 66.Morgan R.A., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans L.S., et al. Expression of chimeric granulocyte-macrophage colony-stimulating factor/interleukin 2 receptors in human cytotoxic T lymphocyte clones results in granulocyte-macrophage colony-stimulating factor-dependent growth. Hum. Gene Ther. 1999;10:1941–1951. doi: 10.1089/10430349950017301. [DOI] [PubMed] [Google Scholar]
- 68.Topp M.S., et al. Restoration of CD28 expression in CD28- CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. . J. Exp. Med. 2003;198:947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rufer N., et al. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. . Blood. 2001;98:597–603. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- 70.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 71.Levine B.L., et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montini E., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. . Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 73.Mauri D., Wyss-Coray T., Gallati H., Pichler W.J. Antigen-presenting T cells induce the development of cytotoxic CD4+ T cells: I. Involvement of the CD80-CD28 adhesion molecules. . J. Immunol. 1995;155:118–127. [PubMed] [Google Scholar]
- 74.Cheever M.A., Greenberg P.D., Fefer A., Gillis S. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. . J. Exp. Med. 1982;155:968–980. doi: 10.1084/jem.155.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganss R., Ryschich E., Klar E., Arnold B., Hammerling G.J. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. . Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 76.Chakraborty M., et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 77.Machiels J.P., et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 78.Obeid M., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 79.Chen M.L., et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pardoll D., Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat. Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 81.Lotze M.T., Line B.R., Mathisen D.J., Rosenberg S.A. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J. Immunol. 1980;125:1487–1493. [PubMed] [Google Scholar]
- 82.Heslop H.E., Rooney C.M. Adoptive cellular immunotherapy for EBV lymphoproliferative disease. Immunol. Rev. 1997;157:217–222. doi: 10.1111/j.1600-065x.1997.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 83.O’Reilly R.J., et al. Biology and adoptive cell therapy of Epstein-Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol. Rev. 1997;157:195–216. doi: 10.1111/j.1600-065x.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 84.Takayama T., et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 85.Bruix J., Boix L., Sala M., Llovet J.M. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 86.Kircher M.F., et al. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. . Cancer Res. 2003;63:6838–6846. [PubMed] [Google Scholar]
- 87.Levine B.L., et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat. Med. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 88.Greenberg P.D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 89.Waller E.K., et al. Irradiated donor leukocytes promote engraftment of allogeneic bone marrow in major histocompatibility complex mismatched recipients without causing graft-versus-host disease. . Blood. 1999;94:3222–3233. [PubMed] [Google Scholar]
- 90.Zhang H., et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat. Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 91.Ku C.C., Murakami M., Sakamoto A., Kappler J., Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. . Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 92.Lake R.A., Robinson B.W. Immunotherapy and chemotherapy — a practical partnership. Nat. Rev. Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 93.Anasetti C., Mule J.J. To ablate or not to ablate? HSCs in the T cell driver’s seat. J. Clin. Invest. 2007;117:306–310. doi: 10.1172/JCI30973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curti B.D., et al. A phase I trial of anti-CD3 stimulated CD4+ T cells, infusional interleukin-2 and cyclophsophamide in patients with advanced cancer. J. Clin. Oncol. 1998;16:2752–2760. doi: 10.1200/JCO.1998.16.8.2752. [DOI] [PubMed] [Google Scholar]
- 95.Ratto G.B., et al. Phase II study of combined immunotherapy, chemotherapy, and radiotherapy in the postoperative treatment of advanced non-small-cell lung cancer. J. Immunother. 2000;23:161–167. doi: 10.1097/00002371-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 96.Ratto G.B., et al. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected nonsmall cell lung carcinoma. Cancer. 1996;78:244–251. doi: 10.1002/(SICI)1097-0142(19960715)78:2<244::AID-CNCR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 97.Atkins M.B., et al. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N. Engl. J. Med. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 98.Kernan N.A., et al. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68:770–773. [PubMed] [Google Scholar]
- 99.Sullivan K.M., et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N. Engl. J. Med. 1989;320:828–834. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- 100.Giralt S., et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 101.Mackinnon S., et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 102.Klebanoff C.A., Khong H.T., Antony P.A., Palmer D.C., Restifo N.P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edinger M., et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 104.Godfrey W.R., et al. In vitro expanded human CD4+CD25+ T regulatory cells markedly inhibit allogeneic dendritic cell stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 105.Hoffmann P., Eder R., Kunz-Schughart L.A., Andreesen R., Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 106.van der Bruggen P., et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 107.Pardoll D.M. Inducing autoimmune disease to treat cancer. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rammensee H.G., Weinschenk T., Gouttefangeas C., Stevanovic S. Towards patient-specific tumor antigen selection for vaccination. . Immunol. Rev. 2002;188:164–176. doi: 10.1034/j.1600-065x.2002.18815.x. [DOI] [PubMed] [Google Scholar]
- 109.Parmiani G., De F.A., Novellino L., Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J. Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 110.Sjoblom T., et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 111.De A.K., et al. Selective activation of peripheral blood T cell subsets by endotoxin infusion in healthy human subjects corresponds to differential chemokine activation. J. Immunol. 2005;175:6155–6162. doi: 10.4049/jimmunol.175.9.6155. [DOI] [PubMed] [Google Scholar]
- 112.Sutmuller R.P., et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barber D.L., et al. Restoring function in exhausted CD8 T cells during chronic viral infection. . Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 114.Kim J.A., et al. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–2037. [PubMed] [Google Scholar]
- 115.Renner C., et al. Cure of disseminated xenografted human Hodgkin’s tumors by bispecific monoclonal antibodies and human T cells: the role of human T-cell subsets in a preclinical model. Blood. 1996;87:2930–2937. [PubMed] [Google Scholar]
- 116.Grabert R.C., et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin. Cancer Res. 2006;12:569–576. doi: 10.1158/1078-0432.CCR-05-2005. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki E., et al. Soluble type II transforming growth factor-beta receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin. Cancer Res. 2004;10:5907–5918. doi: 10.1158/1078-0432.CCR-03-0611. [DOI] [PubMed] [Google Scholar]
- 118.Hu H.M., Winter H., Urba W.J., Fox B.A. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J. Immunol. 2000;165:4246–4253. doi: 10.4049/jimmunol.165.8.4246. [DOI] [PubMed] [Google Scholar]
- 119.Wang L.X., Shu S.Y., Plautz G.E. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65:9547–9554. doi: 10.1158/0008-5472.CAN-05-1175. [DOI] [PubMed] [Google Scholar]
- 120.Turk M.J., et al. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Childs R.W., et al. Successful treatment of metastatic renal cell carcinoma with a nonmyeloablative allogeneic peripheral-blood progenitor-cell transplant: evidence for a graft-versus-tumor effect. J. Clin. Oncol. 1999;17:2044. doi: 10.1200/JCO.1999.17.7.2044. [DOI] [PubMed] [Google Scholar]
- 122.Kausche S., et al. Superior antitumor in vitro responses of allogeneic matched sibling compared with autologous patient CD8(+) T cells. Cancer Res. 2006;66:11447–11454. doi: 10.1158/0008-5472.CAN-06-0998. [DOI] [PubMed] [Google Scholar]
- 123.Bergmann-Leitner E.S., Abrams S.I. Differential role of Fas/Fas ligand interactions in cytolysis of primary and metastatic colon carcinoma cell lines by human antigen-specific CD8+ CTL. . J. Immunol. 2000;164:4941–4954. doi: 10.4049/jimmunol.164.9.4941. [DOI] [PubMed] [Google Scholar]
- 124.Kershaw M.H., et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 125.Sabel M.S., Arora A., Su G., Chang A.E. Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology. 2006;53:360–366. doi: 10.1016/j.cryobiol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Serafini P., et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. . J. Exp. Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang B., et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Irvine K.R., Rao J.B., Rosenberg S.A., Restifo N.P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J. Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenberg S.A., et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst. 1993;85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 130.Graham S., et al. The use of ex vivo-activated memory T cells (autolymphocyte therapy) in the treatment of metastatic renal cell carcinoma: final results from a randomized, controlled, multisite study. Semin. Urol. 1993;11:27–34. [PubMed] [Google Scholar]
- 131.Law T.M., et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832. doi: 10.1002/1097-0142(19950901)76:5<824::aid-cncr2820760517>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]