Abstract
Faithful protein synthesis relies on a family of essential enzymes called aminoacyl-tRNA synthetases, assembled in a piecewise fashion. Analysis of the completed archaeal genomes reveals that all archaea that possess asparaginyl-tRNA synthetase (AsnRS) also display a second ORF encoding an AsnRS truncated from its anticodon binding-domain (AsnRS2). We show herein that Pyrococcus abyssi AsnRS2, in contrast to AsnRS, does not sustain asparaginyl-tRNAAsn synthesis but is instead capable of converting aspartic acid into asparagine. Functional analysis and complementation of an Escherichia coli asparagine auxotrophic strain show that AsnRS2 constitutes the archaeal homologue of the bacterial ammonia-dependent asparagine synthetase A (AS-A), therefore named archaeal asparagine synthetase A (AS-AR). Primary sequence- and 3D-based phylogeny shows that an archaeal AspRS ancestor originated AS-AR, which was subsequently transferred into bacteria by lateral gene transfer in which it underwent structural changes producing AS-A. This study provides evidence that a contemporary aminoacyl-tRNA synthetase can be recruited to sustain amino acid metabolism.
Accurate protein translation requires a complete set of 20 species of perfectly paired aminoacyl-tRNAs (aa-tRNAs). It was therefore expected that each organism should possess 20 aa-tRNA synthetases (aaRSs), each capable of matching a particular amino acid (aa) to the cognate tRNA (reviewed in ref. 1). However, the vast number of sequences that emerged from genome sequencing and biochemical investigations revealed anomalies that forced a revision of this assumption (2). Archaea and various eubacteria are deprived of glutaminyl-tRNA synthetase and more exceptionally of asparaginyl-tRNA synthetase (AsnRS). The homologous aa-tRNAs are formed indirectly by amidation of Glu and Asp, respectively, mischarged on tRNAGln and tRNAAsn by a glutamyl-tRNA synthetase (GluRS) or an aspartyl-tRNA synthetase (AspRS) of relaxed specificity (3–6). In bacteria of the Thermus/Deinococcus group, deprived of the enzymes forming free asparagine (Asn), the indirect route to tRNA asparaginylation also constitutes the sole pathway of Asn biosynthesis (6, 7).
The functional interrelation between the direct and the indirect pathways of amide aa-tRNA formation is not well understood, and the phylogenetic relationship of their partners has not been explored. Emergence of the direct pathways of tRNA glutaminylation and asparaginylation had to be accompanied by concomitant apparition of the enzymes forming the free aa and the homologous aa-tRNA. The paths leading to the emergence of glutaminyl-tRNA synthetase and AsnRS have been documented (8, 9), but the origin of the Gln- and Asn-forming enzymes remains unclear. In particular, it is not known whether the aaRS and the enzyme forming the cognate free aa originated from the same ancestor.
The microbial genomes are sprinkled with polypeptide chains consisting of one of the functional domains of aaRSs (10). In some cases, the participation of these domains in a variety of cellular processes, such as translation regulation (11), stimulation of DNA polymerase processivity (12), and aa synthesis (13), could be assessed. Analysis of the aaRS ORFs in archaeal genomes drew our attention to the fact that all archaea that possess AsnRS (7 of 13) display a second ORF highly homologous to the catalytic core of AsnRS (AsnRS2) but lacking the anticodon-binding domain. We analyzed the functional properties of AsnRS2 from Pyrococcus abyssi, a hyperthermophilic archaeon. The results show that this truncated AsnRS does not form Asn-tRNAAsn but produces Asn by amidation of Asp. Phylogeny suggests this asparagine synthetase (AS) appeared recently in archaea and allowed emergence of a direct and autonomous pathway of tRNA asparaginylation. This study describes an example of emergence of a new tRNA aminoacylation specificity and of the enzyme forming the homologous aa from a contemporary aaRS.
Materials and Methods
General. Escherichia coli ER strain (asnA–, asnB–) was from Genetic Stock Center (Yale University, New Haven, CT), and P. abyssi DNA was a gift from J.-C. Thierry (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France). The pKKET vector is a modified version of pKK223-3 (Amersham Pharmacia) in which the EcoRI site was replaced by the NdeI site. Thermus thermophilus tRNAAsp, tRNAAsn, and AspRS2 were purified from E. coli overproducing strains.
Cloning of the P. abyssi asnS2 Gene. The ORF was amplified by PCR of genomic DNA with 22- and 20-nt-long sense and antisense primers extended by NdeI and PstI or KpnI restriction sites for cloning of the genes in pKKET and pET vectors, respectively. AsnRS2 was expressed in E. coli ER strain (ER/pKKET-asnS2) and in BL21-CodonPlus-RIL strain (BL21/pET-asnS2).
Overexpression and Purification of AsnRS2. The BL21/pET-asnS2 strain was grown overnight at 37°C in LB medium containing ampicillin, and AsnRS2 was expressed by induction with isopropyl β-d-thiogalactoside. The cells were disrupted by sonication; the thermolabile proteins were flocculated by heat treatment of the 105,000 × g supernatant during 30 min at 70°C and removed by centrifugation. AsnRS2 was further purified by chromatographies on DEAE-cellulose and hydroxyapatite followed by FPLC chromatography on an UNO-Q6 (Bio-Rad) column. From 27 g of cells, 80 mg of pure protein was isolated.
AsnRS2 Activities. ATP–PPi exchange. The reaction mixture contained 100 mM NaHepes (pH 7.2), 10 mM MgCl2, 2 mM [32P]PPi, 2 mM ATP, 5 mM l-Asp (0.05–1.35 mM for Km measurements) or l-Asn, and 1 μM P. abyssi AsnRS2. For Ki measurements, the concentration of Asn varied from 12 to 95 μM, and Asp was fixed at 88, 176, or 293 μM. The [32P]ATP that formed after 2–10 min at 70°C was determined in 20-μl aliquots (6).
Aminoacylation. The reaction mixture contained 100 mM NaHepes (pH 7.2), 30 mM KCl, 10 mM MgCl2, 10 μM l-[14C]Asp or l-[3H]Asn, 320 μM unfractionated E. coli tRNA or 30 μM enriched Thermus thermophilus tRNAAsp or tRNAAsn, and 0.1 μM P. abyssi AsnRS2 or Thermus thermophilus AspRS2. The aa-tRNA formed after 1–30 min at 50°C was determined in 40-μl aliquots (6).
Amidation. The reaction mixture contained 100 mM NaHepes (pH 7.2), 10 mM MgCl2,10mMNH4Cl, l-Asn or l-Gln, 10 mM ATP, 0.5 mM l-Asp (0.5–10 mM for Km measurements), and 2 μM AsnRS2. The reaction was conducted at 70°C. For characterization of Asn, [14C]Asp was used and 4.5-μl aliquots were transferred in 1 μl of acetic acid after a 5-min to 3-h incubation. For characterization of the ATP products, the reaction mixture contained 40 μM [γ- or α-32P]ATP, 40 μM l-Asp, and 0.6 μM AsnRS2; after a 10-min to 5-h incubation, 20-μl aliquots were diluted in 80 μl of water; after phenol/chloroform and chloroform extractions, the aqueous phase was dried and the products were suspended in 20 μl of water. The reaction products were analyzed by TLC on cellulose plates of 0.5-μl aliquots. Asp and Asn were separated with the solvent 2-propanol/formic acid/acetic acid/water, 80/10/10/4 (vol/vol/vol/vol); ATP was separated from its products with the solvent isobutyric acid/25% ammonia/water, 50/1.1/28.9 (vol/vol/vol). The labeled products were revealed by image plate with a Fuji Bioimager. The phenylthiohydantoin derivatized products were separated on a Brownlee PTC, C-18, 5-μm column (220 × 2.1 mm2) and analyzed on an Applied Biosystems 420A Derivatizer.
Phylogenetic Analysis. Alignments were performed by using the CLUSTALX program, Version 1.81 (14), and refined with the 3D structures of the proteins (Protein Data Bank). The trees were constructed from a bootstrap of 500 replicates obtained from analysis of an ungapped alignment of 215 residues inferred by the neighbor-joining method by using the PHYLIP package, Version 3.57c (J. Felsenstein, University of Washington, Seattle), with the Dayhoff PAM matrix; they were reconstructed with the maximum-likelihood method by using the PUZZLE program, Version 4.0.2 (15). Trees were edited with the TREEVIEW program, Version 1.5.
Results
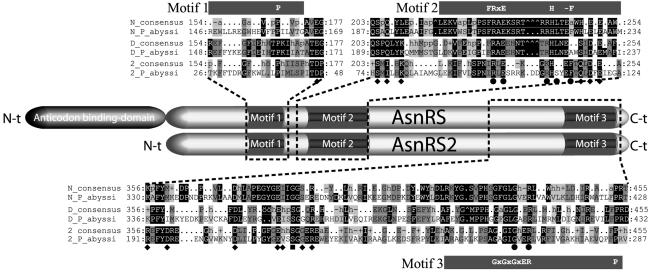
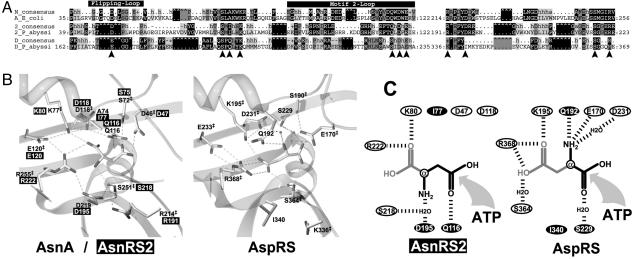
Existence of Two Genes Encoding AsnRS in P. abyssi. Analysis of the annotated genome from P. abyssi reveals two ORFs related to AsnRS, one encoding the full-length enzyme and the other one, a shorter isoform, identified as AsnRS2 sharing 39% identity with the canonical AsnRS. The gene encoding this truncated AsnRS (asnS2) was found in six other archaeal genomes (16) but not in bacterial or eukaryal genomes. Surprisingly, all archaea that encode AsnRS2 also possess the full-length AsnRS (Table 1). Alignment of AsnRS2 with the archaeal AsnRS and AspRS shows this isoform lacks the 100 first aa, which in other AsnRS are organized in the anticodon-binding domain (Fig. 1); nevertheless, the catalytic core displays the three consensus motifs characterizing class II aaRS in which the invariant and semi-invariant residues are mostly conserved (17). To assign a function to these truncated AsnRS, we expressed P. abyssi AsnRS2 in E. coli and analyzed its properties.
Table 1. Occurrence of AsnRS2 and enzymes involved in Asn and Asn-tRNAAsn synthesis in archaea.
| Organism | asnA* | asnB* | gatCAB*† | asnS† | asnS2 |
|---|---|---|---|---|---|
| Crenarchaeota | |||||
| Aeropyrum pernix | - | + | + | - | - |
| Sulfolobus solfataricus | - | - | + | - | - |
| Sulfolobus tokodaii | - | + | + | - | - |
| Pyrobaculum aerophilum | - | + | - | + | + |
| Euryarchaeota | |||||
| Archaeoglobus fulgidus | - | + | + | - | - |
| Halobacterium sp. NRC1 | - | + | + | - | - |
| Methanobacterium thermoautotrophicum | - | + | + | - | - |
| Methanococcus jannaschii | - | + | + | - | - |
| Methanosarcina barkeri | - | + | + | - | - |
| P. abyssi | - | + | - | + | + |
| Pyrococcus furiosus | - | + | - | + | + |
| Pyrococcus horikoshii | - | + | - | + | + |
| Ferroplasma acidarmanus | - | + | - | + | + |
| Thermoplasma acidophilum | - | + | - | + | + |
| Thermoplasma volcanium | - | - | - | + | + |
Only archaeal genome sequences for which the presence (+) or absence (-) of the genes could be ascertained are listed. Genes encoding AsnRS2 (asn2) and the enzymes involved in either Asn (*) or Asn-tRNAAsn (†) formation, asnA, asnB, gatCAB, and asnS encoding, respectively, asparagine synthetases A and B, amidotransferase (AdT), and AsnRS are presented.
Fig. 1.
Alignments of AsnRS2 with archaeal AsnRS and AspRS. The alignment of 7 archaeal AsnRS (N),19 archaeal AspRS (D), and 7 AsnRS2 (2) is summarized. The sequences of the enzymes of P. abyssi (P_abyssi) and the consensus sequences encompassing the consensus motifs 1, 2, and 3 of class II aaRSs are shown. The motifs are schematized by black boxes located at the top or bottom of the sequences, and the invariant residues are indicated in white letters. The percentage homology is symbolized by shading: black and dark-gray shadings denote 100% and 80% of homology, and light-gray shading denotes conservation of the chemical nature in at least 80% of residues; dots symbolize lack of a residue in the detailed sequences and homology lower than 80% in the consensus sequences. Nomenclature of the consensus sequences is the following: a, aromatic; h, hydrophobic; – and +, negatively and positively charged; p, polar or small residues; ^ refers to a gap. Residues interacting with the aa (♦) or the adenylate (•) moiety of Asp∼AMP or with both (▪) in the 3D structure of the complex of Pyrococcus AspRS (22) are indicated.
Cloning, Expression, and Analysis of Substrate Specificity of P. abyssi AsnRS2. Analysis of the extract of the BL21/asnS2 strain showed presence of a thermostable protein corresponding to the 294-aa-long polypeptide chain (molecular mass of 36,061 Da) encoded by asnS2. Gel filtration of the purified protein revealed a molecular mass of 60 kDa, whereas SDS/PAGE showed polypeptide chains of 34 kDa, suggesting a homodimeric structure for the native protein.
Aminoacylation assays on either unfractionated E. coli tRNA or enriched Thermus thermophilus tRNAAsn were unsuccessful with AsnRS2 under conditions where Thermus thermophilus AsnRS forms Asn-tRNA (not shown). Further, AsnRS2 was also unable to form asparaginyl adenylate (Asn∼AMP), as revealed by the absence of Asn-dependent ATP–PPi exchange (not shown). Surprisingly, the enzyme stimulated the exchange in the presence of Asp with a rate constant comparable to that of AspRS2 from Thermus thermophilus, an archaeal-type AspRS (respectively 1.0 and 1.75 s–1 at 70°C), demonstrating its capacity to form Asp∼AMP. However, AsnRS2 was unable to aspartylate tRNA. Despite its inability to activate Asn, AsnRS2 binds Asn, which acts as a competitive inhibitor of Asp (not shown). Affinity of AsnRS2 for Asn exceeds 10 times that for Asp (Km for Asp is 180 μM and Ki for Asn is 16 μM), contrasting with the strict specificity of AspRS and AsnRS for their cognate aa.
Characterization of the Reaction Catalyzed by AsnRS2. Inability of
AsnRS2 to aspartylate tRNA narrowed the possible role for this protein down to
AS or argininosuccinate synthase, which, like AsnRS2, use Asp and ATP as
substrates and form Asp∼AMP. Inspection of the biochemical background of
archaea shows that all are missing asnA, the gene that encodes, in
eubacteria, the ammonia-dependent AS-A
(Table 1). However, with two
exceptions, all archaea possess the glutamine-dependent AS-B; half of them
possess also the AdT able to catalyze the tRNA-dependant Asn formation,
whereas the other half encode AsnRS2 (Table
1). We therefore hypothesized that AsnRS2 might be an enzyme
catalyzing the ammonia-dependent formation of free Asn.
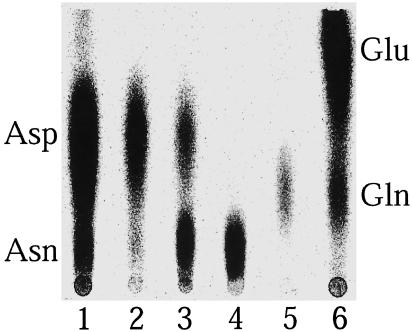
Fig. 2 shows that AsnRS2 was
able, in the presence of 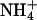 , to
convert Asp into a component of the same chromatographic mobility as Asn (lane
3). Formation of Asn was confirmed by (i) derivatization of the
compounds of the reaction mixture with phenylisothiocyanate, showing formation
of phenylthiohydantoin-Asn (not shown) and (ii) transfer of the
radiolabeled aa formed in the amidation reaction and isolated by TLC, onto
tRNA by Thermus thermophilus AsnRS, which exclusively charges tRNA
with Asn (data not shown).
, to
convert Asp into a component of the same chromatographic mobility as Asn (lane
3). Formation of Asn was confirmed by (i) derivatization of the
compounds of the reaction mixture with phenylisothiocyanate, showing formation
of phenylthiohydantoin-Asn (not shown) and (ii) transfer of the
radiolabeled aa formed in the amidation reaction and isolated by TLC, onto
tRNA by Thermus thermophilus AsnRS, which exclusively charges tRNA
with Asn (data not shown).
Fig. 2.
Identification of the end products formed by P. abyssi AsnRS2 by TLC. Phosphor images of TLC plates of the amidation mixture containing [14C]Asp without amide group donor (lane 2) or with NH4Cl (lane 3) or containing unlabeled Asp and either [3H]Asn (lane 4) or [3H]Gln (lane 5); lanes 1 and 6, controls with labeled aa.
Selectivity of AsnRS2 for free ammonia as an amide group donor (Fig. 2, lanes 2 and 3) and absence of glutaminase or asparaginase activities able to produce free NH3 (lanes 4 and 5) reveal a mechanistic resemblance to AS-A (18).
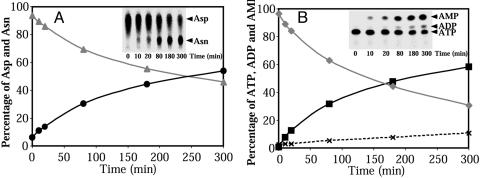
Formation of Asn is correlated with conversion of ATP into AMP
(Fig. 3). Quantification of the
end products along the kinetic curves shows formation of Asn and AMP in a 1/1
stoichiometry. However, consumption of ATP exceeds formation of AMP
(Fig. 3B) because of
an accumulation of a small amount of ADP. This side-product appears only under
amidation conditions and represents ≈8% of the ATP consumed. A
Km for Asp of 4–5 mM and a kcat
of 2–3 s–1 were determined. The affinity for
 was high because the contaminating
ions partially stimulated the amidation. Finally, among the various nucleoside
triphosphates only ATP promotes amidation.
was high because the contaminating
ions partially stimulated the amidation. Finally, among the various nucleoside
triphosphates only ATP promotes amidation.
Fig. 3.
Kinetics of substrate consumption and of end-product formation by AsnRS2. (A) Consumption of [14C]Asp (▴) and formation of [14C]Asn (•). (B) Consumption of [α-32P]ATP (♦) and formation of [32P]AMP (▪) and [32P]ADP (×). The labeled reactants were fractionated by TLC (Inset) and quantified by using the IMAGE GAUGE software (Fuji), and their amounts are expressed as the percentage of the total radioactivity in the assay.
P. abyssi AsnRS2 Confers Asn Prototrophy. To unambiguously prove that AsnRS2 ensures Asn synthesis in vivo, we attempted complementation of the E. coli ER strain, auxotrophic for Asn (19), by transformation with pKKET-asnS2. Fig. 4 shows that P. abyssi asnS2 was able to confer Asn prototrophy to the transformed E. coli strain. This result constitutes strong evidence that AsnRS2 serves as AS-A in P. abyssi.
Fig. 4.
Complementation of the Asn auxotrophy of E. coli ER strain by the P. abyssi asnS2 gene. The E. coli ER strain was transformed with either the pKKET vector or the recombined pKKET-asnS2 vector and grown on minimal M9 medium agar plates supplemented with ampicillin, and 0.5 mM isopropyl β-d-thiogalactoside in the presence (+) or absence (–) of 40 μg/liter l-Asn.
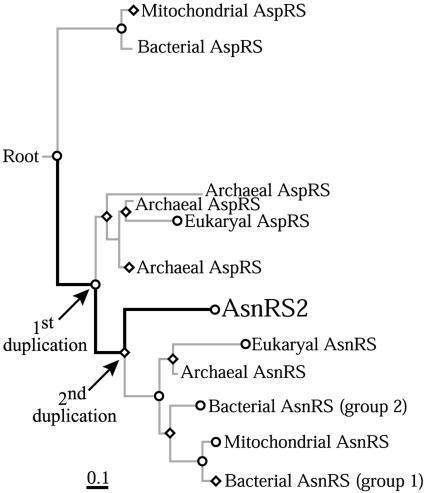
Retracing Appearance and Evolution of the Autonomous tRNA Asparaginylation Pathway. The tree reconstructed by alignment of AsnRS2 with AspRS and AsnRS sequences shows two main groupings, one constituted by the bacterial AspRS and the other one in which archaeal and eukaryal AspRS are intermixed with AsnRS and AsnRS2 (Fig. 5). Branching of the AsnRS2 clade suggests that this enzyme derives from an AspRS ancestor that also evolved AsnRS and the archaeal/eukaryal AspRS. The most probable event that could explain apparition of AsnRS2 would be two gene duplications (Fig. 5). The first was that of the AspRS ancestor, from which one copy evolved into archaeal/eukaryal AspRS and the other one into AsnRS. The second was that of AsnRS, from which one copy originated AsnRS2 and the other one conserved the function of AsnRS. The fact that AsnRS is present in the three phyla whereas AsnRS2 is archaeal-specific suggests that AsnRS appeared just before the split of archaea and eukaryotes and AsnRS2 after the split. The high bootstrap values obtained along the evolutionary path leading to AsnRS2 (in thick bars) supports this scenario. Because the topology of the trees was independent of the method used, bias inherent to the use of a particular method was minimized.
Fig. 5.
Attempt to pinpoint the origin of the archaeal ammonia-dependent Asn metabolism. The tree is based on an ungapped alignment done by CLUSTALX of 7 AsnRS2, 60 AspRS, 49 AsnRS, and 61 lysyl-tRNA synthetase (LysRS) sequences. LysRS sequences were used to root the tree. Nodes statistically relevant [bootstrap value of 100% (⋄) or >80% (○)] are indicated. The evolutionary path of AsnRS2 is indicated by thick bars. The arrows indicate the gene duplications. The scale bar represents 10 substitutions per 100 aa.
Because of the absence of significant sequence similarity of AS-A with AspRS and AsnRS, introduction of AS-A sequences in the alignment resulted in (i) a significant sensitivity of the topology of the trees to the method used for their reconstruction and (ii) a drop of ≈50% of the bootstrap values of all branches (data not shown). Nevertheless, the AS-A clade was always branched to the AsnRS2 lineage, suggesting that bacteria did not evolve AS-A but acquired this enzyme by lateral gene transfer from archaea. This event is supported by the conservation of a synteny involving asnA in bacteria or asnS2 in archaea and the gene encoding a transcription regulator (asnC). This synteny is found in 6 of 12 species of bacteria possessing AS-A (20) and in two archaea.
Mapping AsnRS2 Active Site. The alignments of AsnRS2 with AS-A (Fig. 6A) refined by the 3D structure of E. coli AS-A complexed with AMP and Asn (21) allowed identification of the essential residues of P. abyssi AsnRS2 and mapping them in the active site of E. coli AS-A. All residues of AS-A contacting the Asn substrate are strictly conserved at almost the same position in AsnRS2 with only one exception (A74 in AS-A replaced by I77 in AsnRS2, Fig. 6 A and B). The same strategy was used to map AsnRS2 residues (Figs. 1 and 6A) in the 3D structure of the active site of Pyrococcus AspRS complexed with Asp∼AMP (22). Most of the residues conserved in the active sites of AS-A, AsnRS2, and contacting Asn are conserved in AspRS and contact Asp. Indeed, of the 11 residues involved in recognition of Asn in AS-A (and in AsnRS2) and of Asp in AspRS, 8 are strictly conserved but differ in the interactions they make with the substrate. Additionally, of the 8 residues involved in recognition of AMP in the AspRS complex (23), 6 are conserved in AsnRS2 and AS-A and contact AMP (Fig. 1). However, the 3 residues contacting the α-COOH, α-NH2, and β-CO groups of the aa substrate are not conserved (Fig. 6B). In AsnRS2, D195, equivalent to D219 in AS-A and involved in recognition of the α-NH2 group of the substrate, is replaced in Pyrococcus AspRS by I340. In the second strand of motif 2 of Pyrococcus AspRS, S229, which binds the α-COOH group of Asp with a water-mediated bond, is replaced by Q116 in both AsnRS2 and AS-A, which now interact with the β-COOH group of the substrate. Finally, Q192, the last residue of the QSPQ motif of AspRS, which contacts the α-NH2 group of Asp, is replaced in AS-A and AsnRS2 by A74 and I77 and does not interact with the substrate. Nonconservation in AsnRS2 (or AS-A) and AspRS of the residues contacting identical substrate groups provides the structural basis for the reversed orientation of the Asp substrate in the active centers (Fig. 6C), and suggests that conversion of the α-COOH-activating site of AspRS into the β-COOH-activating site of AS-A or AsnRS2 was achieved by only three substitutions: I340 to D195, Q192 to I77, and S229 to Q116 (Fig. 6C).
Fig. 6.
Alignment-based identification of P. abyssi AsnRS2 active site residues. (A) Comparative alignment of AS-A (A), AsnRS2 (2), and AspRS (D). Only blocks of sequences encompassing the active-site residues involved in binding of Asp (arrowheads) are shown. The equivalent of AspRS flipping- and motif 2-loops are indicated by black boxes located at the top of the alignment. Nomenclature for the alignment is indicated in the Fig. 1 legend. (B) Comparison of the 3D structures of Pyrococcus AspRS and E. coli AS-A active sites. Coordinates of AspRS complexed to Asp∼AMP (22) and of AS-A complexed to Asn and ATP (21) were used. Hydrogen bonds are symbolized by dashed lines and water molecules by small spheres, and ‡ refers to residues of the same chemical nature spatially conserved in AspRS and AS-A active sites. Residues in white letters over black boxes are those of P. abyssi AsnRS2 identified in the alignment with AS-A shown in A as potential candidates for recognition of Asp. (C) Schematized 2D representation comparing recognition of Asp by Pyrococcus AspRS and AsnRS2. The residues interacting with Asp in AspRS and suggested to interact with Asp in AsnRS2 are displayed. Residues in ellipses are spatially conserved in AspRS and AsnRS2; residues in white letters in black ellipses determine different orientations of Asp in the two active sites; hydrogen bonds are symbolized by dashed lines.
The active sites of AS-A and AsnRS2 differ additionally from that of AspRS by the flipping and motif 2 loops that might be related to differences in the reactions they catalyze: AS-A and AsnRS2 transfer the activated Asp onto ammonia and AspRS onto tRNAAsp. Comparison of the 3D structures of E. coli AS-A and Pyrococcus AspRS and alignments with AsnRS2 show conservation of an acidic residue in the flipping loops, Glu in AspRS (E170 in Pyrococcus AspRS) and Asp in AS (D46 in AS-A and D47 in AsnRS2), both contacting by hydrogen bond the α-NH2 group of the substrate (Fig. 6B). In addition, the flipping and motif 2 loops differ in size. In AS-A the flipping loop is five residues longer than in AspRS and AsnRS2, whereas in AsnRS2 the motif 2 loop is longer than in AspRS and in AS-A. These differences probably reflect distinct functionalities of AS (AS-A and AsnRS2) and AspRS. The flipping loop, which entraps Asp∼AMP in the active site of AspRS (22, 23) and flips back in its open position upon binding of the cognate tRNAAsp to allow the nucleophilic attack of the activated α-COOH by tRNA, might entrap Asp∼AMP and the ammonia substrate in AS. Motif 2 loop, which in AspRS is involved in ATP binding and establishes base-specific hydrogen bonds with G73 and C74 of the tRNA acceptor stem (23), might prevent recognition of tRNA by AS.
Discussion
This investigation proves that AsnRS2 is the archaeal orthologue of the bacterial ammonia-dependent AS-A. Therefore we renamed this protein archaeal AS-A (AS-AR). Previous studies suggested that AS-A might have evolved from an archaeal/eukaryal ancestor (24, 25). This hypothesis was supported by the structural resemblance at the 3D level between E. coli AS-A and the catalytic core of yeast AspRS (21). However, the lack of significant sequence similarities did not allow us to establish the phylogeny between these enzymes. We show that AS-AR constitutes the link in the evolutionary path that led to emergence of ammonia-dependent Asn synthesis. An AspRS ancestor originated AS-AR through two gene duplication events, one in the archaeal/eukaryal progenitor and another one later in archaea. The phylogenetic analysis suggests that bacterial AS-A results from a lateral gene transfer of archaeal AS-AR. Thus the absence of sequence homology of archaeal AspRS with AS-A, contrasting with their high degree of identity with AS-AR, suggests that whereas the gene in bacteria underwent structural changes, the archaeal gene, for reasons that would have to be clarified, remained untouched. A similar phenomenon occurred in the evolutionary history of bacterial AsnRSs, which segregate in two subgroups, one typically bacterial (Fig. 5, group 1) and another one archaeal-like (Fig. 5, group 2).
Three prerequisites were needed for evolving AS from AspRS: (i) switch in activation of the α-COOH group of the Asp substrate (for AspRS) to the β-COOH group (for AS), (ii) loss of transfer of the β-COOH-activated Asp onto tRNA, and (iii) binding of ammonia and transfer onto the activated β-COOH group of Asp. Acquisition by AspRS of the capacity to activate this COOH group had to be accompanied by the loss of tRNA aminoacylation to prevent isopeptide bond formation in proteins and premature termination of peptide elongation on ribosomes. Further, because the activated Asp can react with nucleophilic acceptors (26), loss of the tRNA charging capacity of the β-COOH-activating AspRS had to be correlated with acquisition of the amidation reaction to prevent release of Asp∼AMP.
So far, four aaRSs truncated from their anticodon-binding domain have been described: GenX (27), BirA (28), HisZ (13), and AlaX (10), respectively homologues of Lys-, Ser-, His-, and AlaRS. Their cellular functions are diverse, and they are distributed in all kingdoms of life. HisZ functionally most resembles AS-AR. Although this enzyme does not form aa directly, it constitutes the functional subunit of the ATP-phosphoribosyltransferase, involved in the His biosynthetic pathway (13). Thus, to our knowledge, AS-AR constitutes the first example of a truncated aaRS capable of synthesizing its cognate aa.
Absence of anticodon-binding domain raises the question whether these isoforms evolved from complete aaRSs that lost their anticodon-binding domain or from simpler aaRS ancestors that had not yet acquired this domain. Because AS-AR evolved from an archaeal AspRS ancestor that also originated AsnRS (Fig. 5), and AspRS and AsnRS both display an anticodon-binding domain organized in an OB-fold (29), the AspRS ancestor had to already possess this domain. Otherwise, one has to consider the highly improbable event that bacterial, archaeal, and eukaryal AsnRS and AspRS independently acquired the same anticodon-binding domain. The loss of this module was probably imposed by a strong selection pressure to destroy aminoacylation capacity of an AspRS activating group other than the α-COOH of Asp. Because in subclass IIb aaRS residues from anticodon constitute the major elements responsible for efficient aminoacylation (reviewed in refs. 30 and 31), loss of the anticodon-binding domain will impair the capacity of these aaRSs to bind and aminoacylate their tRNA substrate.
Synthesis of Asn is distinctive because it can be produced by three different and unrelated enzymes, AS-A (or AS-AR), AS-B and AdT. AS-B and AdT are found in the three domains of life, suggesting that they were already present in the latest common ancestor. AS-A, however, is found only in a subset of pathogenic bacteria and in seven archaea. Furthermore, almost all organisms that possess AS-A also contain AS-B or AdT. Only three organisms, two bacteria (Haemophilus influenzae and Pasteurella multocida) and one archaea, (Thermoplasma volcanium) escape this rule, and strictly depend on AS-A or AS-AR for Asn synthesis. The reason why AS-A predominantly coexists with another AS is unclear. Presence of AS-AR in all archaea that display AsnRS suggests that this Asn synthetase has especially been acquired by archaea to allow emergence of the direct and autonomous pathway of tRNA asparaginylation.
Acknowledgments
We thank E. Schmitt (Ecole Polytechnique, Palaiseau, France) and D. Moras (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France) for the coordinates of the 3D structures of Pyrococcus AspRS complexed to Asp≈AMP and R. Giegé (Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France) for suggestions and critical reading of the manuscript. This work was supported by the Université Louis Pasteur (Strasbourg), the Centre National de la Recherche Scientifique et Technique, and a grant from the Association de la Recherche contre le Cancer. H.R. is a recipient of a fellowship from Ministère de l'Education Nationale de la Recherche et de la Technologie.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: aa, amino acid; aa-tRNA, aminoacyl-tRNA; aaRS, aa-tRNA synthetase; AsnRS, asparaginyl-tRNA synthetase; AspRS, aspartyl-tRNA synthetase; AS, asparagine synthetase; AS-A, bacterial ammonia-dependent AS; AS-AR, archaeal AS-A; AdT, tRNA-dependent amidotransferase.
See commentary on page 9650.
References
- 1.Ibba, M., Francklyn, C. & Cusack, S., eds. (2003) The Aminoacyl-tRNA Synthetases (Landes Bioscience, Georgetown, TX), in press.
- 2.Ibba, M., Becker, H. D., Stathopoulos, C., Tumbula, D. L. & Söll, D. (2000) Trends Biochem. Sci. 25, 311–316. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox, M. & Nirenberg, M. (1968) Proc. Natl. Acad. Sci. USA 61, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curnow, A. W., Hong, K., Yuan, R., Kim, S., Martins, O., Winkler, W., Henkin, T. M. & Söll, D. (1997) Proc. Natl. Acad. Sci. USA 94, 11819–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curnow, A. W., Ibba, M. & Söll, D. (1996) Nature 382, 589–590. [DOI] [PubMed] [Google Scholar]
- 6.Becker, H. D. & Kern, D. (1998) Proc. Natl. Acad. Sci. USA 95, 12832–12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curnow, A. W., Tumbula, D. L., Pelaschier, J. T., Min, B. & Söll, D. (1998) Proc. Natl. Acad. Sci. USA 95, 12838–12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamour, V., Quevillon, S., Dirion, S., N'Guyen, V. C., Lipinski, M. & Mirande, M. (1994) Proc. Natl. Acad. Sci. USA 91, 8670–8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woese, C. R., Olsen, G. J., Ibba, M. & Söll, D. (2000) Microbiol. Mol. Biol. Rev. 64, 202–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schimmel, P. & Ribas De Pouplana, L. (2000) Trends Biochem. Sci. 25, 207–209. [DOI] [PubMed] [Google Scholar]
- 11.Kaniga, K., Compton, M. S., Curtiss, R. & Sundaram, P. (1998) Infect. Immun. 66, 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrodeguas, J. A., Kobayashi, R., Lim, S. E., Copeland, W. C. & Bogenhagen, D. F. (1999) Mol. Cell. Biol. 19, 4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sissler, M., Delorme, C., Bond, J., Ehrlich, S. D., Renault, P. & Francklyn, C. (1999) Proc. Natl. Acad. Sci. USA 96, 8985–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. (1997) Nucleic Acids Res. 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt, H. A., Strimmer, K., Vingron, M. & von Haeseler, A. (2002) Bioinformatics 18, 502–504. [DOI] [PubMed] [Google Scholar]
- 16.Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990) J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- 17.Eriani, G., Delarue, M., Poch, O., Gangloff, J. & Moras, D. (1990) Nature 347, 203–206. [DOI] [PubMed] [Google Scholar]
- 18.Cedar, H. & Schwartz, J. H. (1969) J. Biol. Chem. 244, 4122–4127. [PubMed] [Google Scholar]
- 19.Felton, J., Michaelis, S. & Wright, A. (1980) J. Bacteriol. 142, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolling, R., Gielow, A., Seufert, W., Kucherer, C. & Messer, W. (1988) Mol. Gen. Genet. 212, 99–104. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsu, T., Kato, H. & Oda, J. (1998) Nat. Struct. Biol. 5, 15–19. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt, E., Moulinier, L., Fujiwara, S., Imanaka, T., Thierry, J.-C. & Moras, D. (1998) EMBO J. 17, 5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulinier, L., Eiler, S., Eriani, G., Gangloff, J., Thierry, J.-C., Gabriel, K., McClain, W. H. & Moras, D. (2001) EMBO J. 20, 5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatti, D. L. & Tzagoloff, A. (1991) J. Mol. Biol. 218, 557–568. [DOI] [PubMed] [Google Scholar]
- 25.Hinchman, S. K., Henikoff, S. & Schuster, S. M. (1992) J. Biol. Chem. 267, 144–149. [PubMed] [Google Scholar]
- 26.Kern, D., Lorber, B., Boulanger, Y. & Giegé, R. (1985) Biochemistry 24, 1321–1332. [Google Scholar]
- 27.Kong, L., Fromant, M., Blanquet, S. & Plateau, P. (1991) Gene 108, 163–164. [DOI] [PubMed] [Google Scholar]
- 28.Artymiuk, P. J., Rice, D. W., Poirrette, A. R. & Willet, P. (1995) Nat. Struct. Biol. 1, 758–760. [DOI] [PubMed] [Google Scholar]
- 29.Murzin, A. G. (1993) EMBO J. 12, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giegé, R., Florentz, C., Kern, D., Gangloff, J., Eriani, G. & Moras, D. (1996) Biochimie 78, 605–623. [DOI] [PubMed] [Google Scholar]
- 31.Kern, D., Roy, H. & Becker, H. D. (2003) in The Aminoacyl-tRNA Synthetases, eds. Ibba, M., Francklyn, C. & Cusack, S. (Landes Bioscience, Georgetown, TX), in press.