Abstract
B cell antigen receptors (BCRs) are multimeric transmembrane protein complexes comprising membrane-bound immunoglobulins (mIgs) and Ig-α/Ig-β heterodimers. In most cases, transport of mIgs from the endoplasmic reticulum (ER) to the cell surface requires assembly with the Ig-α/Ig-β subunits. In addition to Ig-α/Ig-β, mIg molecules also bind two ER-resident membrane proteins, BAP29 and BAP31, and the chaperone heavy chain binding protein (BiP). In this article, we show that neither Ig-α/Ig-β nor BAP29/BAP31 nor BiP bind simultaneously to the same mIgD molecule. Blue native PAGE revealed that only a minor fraction of intracellular mIgD is associated with high-molecular-weight BAP29/BAP31 complexes. BAP-binding to mIgs was found to correlate with ER retention of chimeric mIgD molecules. On high-level expression in Drosophila melanogaster S2 cells, mIgD molecules were detected on the cell surface in the absence of Ig-α/Ig-β. This aberrant transport was prevented by coexpression of BAP29 and BAP31. Thus, BAP complexes contribute to ER retention of mIg complexes that are not bound to Ig-α/Ig-β. Furthermore, the mechanism of ER retention of both BAP31 and mIgD is not through retrieval from a post-ER compartment, but true ER retention. In conclusion, BAP29 and BAP31 might be the long sought after retention proteins and/or chaperones that act on transmembrane regions of various proteins.
The antigen receptor on B cells (BCR) is a multiprotein complex comprising one membrane-bound Ig (mIg) molecule and an Ig-α/Ig-β heterodimer (1, 2). A mIg molecule (Fig. 5A) contains two identical membrane-bound heavy chains (belonging to one of the five heavy-chain isotypes, e.g., δm in the case of mIgD) and two identical light chains (λ or κ). Ig-α (CD79a) and Ig-β (CD79b) are related transmembrane (TM) proteins, each of which possesses an extracellular Ig domain, a TM region, and a cytoplasmic tail carrying an immunoreceptor tyrosine-based activation motif (ITAM) (3). On BCR engagement, tyrosine residues of the ITAMs become phosphorylated and contribute to the activation of signaling molecules (4, 5).
Fig. 5.
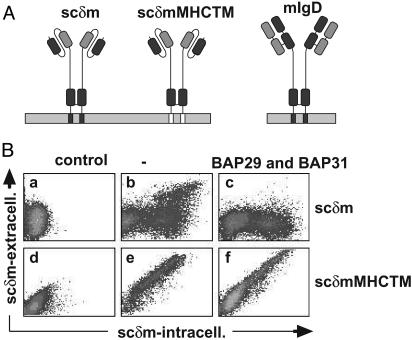
Expression of scδm molecules on the cell surface. (A) Images of scδm, scδmMHCTM, and mIgD molecules are shown. The heavy chain-derived sequence is dark gray, the light chain-derived sequence is light gray, and the TM region of MHCI is white. (B) Untransfected S2 cells (Ba and Bd)orscδm(b) and scδmMHCTM (e) transfectants, as well as BAP29/BAP31/scδm (c) and BAP29/BAP31/scδmMHCTM cotransfectants (f), were stained first on the cell surface (phycoerythrin) and then intracellularly (FITC) with an anti-sc antibody. Cells were analyzed as shown in Fig. 4.
Membrane-proximal Ig domains, as well as TM regions of mIg, play a crucial role in BCR assembly by mediating noncovalent association with Ig-α/Ig-β. TM regions of mIg heavy chains most likely span the membrane as α-helices and comprise 25 amino acid residues, several of which possess polar side chains. One side of their TM α-helix is conserved among different mIg isotypes (1, 6). In the case of mIgM, this side has been shown to bind to Ig-α/Ig-β (7–10). Furthermore, the other side of the mIg TM α-helix contains class-specific amino acids and is involved in the oligomerization of BCRs (11). The cytoplasmic parts of mIgM and mIgD are identical, consisting of only three amino acids.
It was shown that mIg association with Ig-α/Ig-β is necessary for transporting mIgM, mIgA, and mIgE from the endoplasmic reticulum (ER) to the cell surface (12, 13). In contrast, the presence of Ig-α/Ig-β is not required for surface transport of mIgG and, in some cases, of mIgD molecules (13–18). Polar residues within TM regions of mIgs have long been known to play a crucial role in retaining these molecules within the ER if Ig-α and/or Ig-β are absent. Much of our current knowledge about this phenomenon comes from studies on mIgM, in which experimental substitution of polar TM residues for hydrophobic amino acids led to impaired ER retention (reviewed in ref. 6). Although the underlying retention mechanism is not known to date, it apparently involves ubiquitous factors, because mIgM can be efficiently retained in the ER of nonlymphoid cell types like pituitary cells or NIH fibroblasts (13, 19).
We previously reported the copurification of two related TM proteins with mIgD and (to a lesser extent) mIgM molecules. These proteins were termed BCR-associated protein of 29 kDa (BAP29) and 31 kDa (BAP31) (20, 21). The TM region of mIgD is sufficient for binding to BAP proteins, and this interaction involves polar amino acids within the TM region of mIgD (22). BAP31 and BAP29 are ubiquitously expressed polytopic membrane proteins, which are characterized by a hydrophobic N-terminal section with three putative TM regions and a C-terminal cytoplasmic tail containing predicted coiled-coil regions (20, 22, 23). BAP31 and BAP29 possess a C-terminal double-lysine motif (KKXX), which is known to localize proteins to the ER (24). Indeed, ER localization of BAP31 has been reported in non-B cell lines (23, 25).
BAP31 further interacts with the polytopic TM protein cystic fibrosis TM regulator (CFTR) and cellubrevin (25, 26). BAP31 was found to be involved in ER retention of CFTR and export of cellubrevin from the ER.
Initial copurification experiments led us to conclude that BAP proteins are part of the IgD–BCR complex, and that this interaction contributes to signaling differences between IgM- and IgD-BCRs. In this article, we show that neither BAP29 nor BAP31 are subunits of the IgD–BCR complex, but rather they interact with relatively few mIgD molecules in the ER. We further show that these high-Mr BAP29/BAP31 complexes are involved in ER retention of mIg molecules in the absence of Ig-α/Ig-β.
Materials and Methods
DNA Constructs. Expression vectors for Drosophila melanogaster S2 cells were based on the plasmid pRmHa-3, which contains a metallothionein promoter (27). Clonings are included in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Cell Culture. The murine B cell line J558L and its transfectants [J558Lδm/Ig-α (20) and J558Lδm clone 9W] were obtained and cultured as described (22). S2 cells were maintained and transfected as described (28), with the exception being that 1 μg of plasmid DNA was transfected (1 μg of expression vectors for BAP29 and BAP31 was added where indicated) by using Lipofectin (Life Technologies, Grand Island, NY). To induce the metallothionein promoter, 1 mM CuSO4 was added to the cell culture for 24 h. Chinese hamster ovary (CHO) and Cos-7 cells were cultured and transfected as described (29) and according to standard procedures, respectively.
Flow Cytometry. For FACScan (Becton Dickinson) analysis, cells were stained sequentially with an anti-CD4 antiserum (Santa Cruz Biotechnology) and a phycoerythrin-labeled anti-rabbit IgG antiserum. Alternatively, the mAb Ac146 (30), which recognizes the single-chain (sc)δm molecule, and a phycoerythrin-labeled anti-mouse IgG antiserum were used. After surface staining, cells were permeabilized with 0.1% saponin and stained with the same primary antibodies and FITC-labeled secondary antisera. Cells were washed, analyzed with a FACScan (Becton Dickinson), and 104 living cells were plotted on a double-logarithmic scale.
Affinity Purification and Western Blotting. To stimulate J558L transfectants, 2 × 107 cells were incubated with 50 μM pervanadate for 3 min as described (31) and lysed in 1 ml of lysis buffer (22) containing 1% Triton X-100. Phosphorylated proteins were purified with anti-phosphotyrosine agarose (PT66, Sigma). Alternatively, mIg was affinity-purified by using NP (4-hydroxy-3-nitrophenylacetate)-conjugated Sepharose and subsequently eluted with 0.5 mM NIP-cap (5-iodo-4-hydroxy-3-nitrophenylacetate NP coupled to ε-aminocaproic acid) in lysis buffer. For immunoprecipitations, we used an anti-CD4 (Dianova, Hamburg, Germany), or anti-δ and anti-λ (Southern Biotechnologies) antibodies and protein G-coupled Sepharose. Reducing SDS/PAGE and Western blotting are described (22). Western blots were developed with the following antibodies: anti-phosphotyrosine (4G10, Upstate Biotechnology); anti-Ig-β (32); anti-BAP29 and anti-BAP31 (22); anti-Ig-α; anti-hCD4 (Santa Cruz Biotechnology); anti-heavy chain binding protein (BiP) (anti-GRP78, Alexis Biochemicals, San Diego); or horseradish peroxidase-conjugated anti-δ and anti-λ antisera. Secondary antibodies were used as described (20, 22).
Immunofluorescence Microscopy. CHO and Cos-7 cells were grown on glass coverslips at 37°C. For the temperature shift, cells were incubated in a water bath at 15°C for 3 h. Cells were washed twice in PBS and fixed and permeabilized with methanol (–20°C) for 5 min and acetone (–20°C) for 5 sec. Antibody stainings were performed as described (25, 33). Anti-BAP and anti-sc stainings were performed simultaneously on the same sample. Microscopic pictures were recorded by using a Leica confocal microscope. Control experiments were performed to ascertain specificity of the antibody reactions.
Results
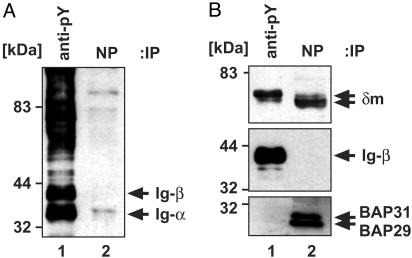
Exclusive Binding of Either the BAP29/BAP31 or the Ig-α/Ig-β Heterodimer to mIgD. Copurification revealed that mIgD binds to both BAP29/BAP31 and Ig-α/Ig-β (20). Here we sought to determine whether BAP proteins and Ig-α/Ig-β bind simultaneously to the same mIgD molecule, or whether they exclude each other in mIgD binding. To distinguish between these possibilities, a two-step purification approach was carried out by using J558Lδm/Ig-α cells that express a complete IgD-BCR specific for the hapten NP. Cells were treated with the phosphatase inhibitor pervanadate allowing phosphorylation of many protein tyrosine kinase substrates including Ig-α and Ig-β (31). Because BAP and mIg proteins are not kinase substrates, they remain unphosphorylated under these conditions. Pervanadate-treated cells were lysed in Triton X-100, a detergent suitable for copurifying Ig-α/Ig-β and BAP complexes with mIgD molecules (34). First, we immunopurified phosphorylated proteins by using anti-phosphotyrosine antibodies. mIgD molecules remaining in the supernatant were subsequently affinity-purified with NP-Sepharose. Proteins were separated by SDS/PAGE and subjected to Western blotting. Detection of proteins was carried out with anti-phosphotyrosine (Fig. 1A) and subsequently with anti-δ, anti-Ig-β, anti-BAP29, and anti-BAP31 antibodies (Fig. 1B). Ig-α/Ig-β became phosphorylated, allowing purification of IgD·Ig-α/Ig-β complexes with anti-phosphotyrosine Sepharose (Fig. 1 A, lane 1). BAP proteins could not be detected in these precipitates (Fig. 1B, lane 1). mIgD molecules purified from the remaining supernatant were bound to the BAP proteins, but not to Ig-α/Ig-β (Fig. 1B, lane 2). A higher-Mr form of δm was copurified with Ig-α/Ig-β (Fig. 1B Top, lane 1), and exhibited resistance to endoglycosidase H (EndoH) treatment (data not shown). In contrast, mIgD purified with NP-Sepharose (Fig. 1B Top, lane 2) contained δm chains of the lower-Mr form and was EndoH sensitive (data not shown). Therefore, the mIgD·Ig-α/Ig-β complexes (BCRs) mainly contained the mature surface form, whereas mIgD·BAP complexes contained the immature ER form of the δm heavy chain. These results demonstrate that BAP29/BAP31 and Ig-α/Ig-β do not bind simultaneously to the same mIgD molecule. BAP proteins are therefore not a part of the BCR complex.
Fig. 1.
Triton X-100-solubilized mIgD complexes exclusively contain either Ig-α/Ig-β or BAP29/BAP31. Phosphotyrosine-containing proteins were purified from stimulated J558Lδm/Ig-α cells (lanes 1), and mIgD complexes remaining in the supernatant were purified with NP-Sepharose (lanes 2). Proteins were separated by SDS/PAGE and detected with anti-phosphotyrosine antibodies (A) and anti-δ, anti-Ig-β, and a mixture of anti-BAP29 and anti-BAP31 antibodies (B).
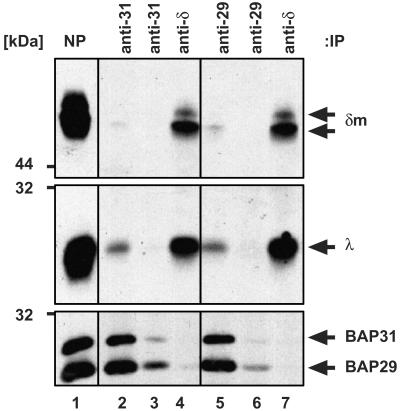
Only a Minor Fraction of Intracellular mIgD Is Bound to BAP29/BAP31. We next investigated whether all intracellular mIgD molecules are associated with the BAP proteins. mIgD complexes from a J558Lδm/Ig-α lysate were first affinity-purified with NP-Sepharose (Fig. 2, lane 1), then were eluted from the washed beads with the free hapten NIP-cap. BAP proteins present in the eluate were repeatedly immunoprecipitated with either an anti-BAP31 (lanes 2 and 3) or an anti-BAP29 antiserum (lanes 5 and 6). Subsequently, the remaining mIgD molecules were purified from the BAP-depleted eluate with an anti-δ antiserum (lanes 4 and 7). Proteins were analyzed by SDS/PAGE and Western blotting. Although all BAP proteins in this analysis were copurified with mIgD by NP-Sepharose (Fig. 2, lane 1), only minor amounts of mIgD (δm and λ) were copurified with anti-BAP31 (lane 2) or anti-BAP29 (lane 5) antisera. The majority of mIgD molecules in the NP eluate was not bound to BAP proteins (lanes 4 and 7). Besides the higher-(Ig-α/Ig-β-associated) Mr form of δm, these mIgD molecules contained the lower-Mr form of δm (lanes 4 and 7). Thus, the majority of intracellular mIgD molecules are not BAP-bound. To rule out the possibility that anti-BAP antibodies possess the ability to disrupt mIgD·BAP29/BAP31 complexes, J558Lδm/Ig-α lysates were incubated with anti-BAP antibodies, after which their copurification with NP-Sepharose-bound mIgD·BAP29/BAP31 was observed (data not shown).
Fig. 2.
Only minor amounts of mIgD are associated with BAP29/BAP31. The mIgD complexes were purified from J558Lδm/Ig-α lysates with NP-Sepharose (lane1), eluted by free hapten, and precipitated twice with either an anti-BAP31 (lanes 2 and 3) or an anti-BAP29 antiserum (lanes 5 and 6). Subsequently, the mIgD molecules remaining in the supernatants were purified with an anti-δ antiserum (lanes 4 and 7). Proteins were separated by SDS/PAGE and detected with anti-δ, anti-λ, anti-BAP29, and anti-BAP31 antisera.
Thus, three populations of mIgD molecules can be discriminated in B cells: a large pool of free mIgD, a minor pool of BAP-associated mIgD, and a pool of Ig-α/Ig-β-associated mIgD. All mIgD-associated BAP31 proteins were copurified with an anti-BAP29 antiserum (lanes 5 and 7), indicating that BAP29 and BAP31 bind simultaneously to the same mIgD molecules. In NIP eluates of J558Lδm/Ig-α lysates, BAP29 and BAP31 are present in roughly equimolar amounts (20). Therefore, each of the few BAP-associated mIgD molecules seems to bind to a high-Mr BAP complex composed of multiple BAP29/BAP31 heterodimers. In Supporting Text we show by using blue native PAGE that the intracellular pool of mIgD molecules exists in different high-Mr complexes that are BAP-bound (see Fig. 7, which is published as supporting information on the PNAS web site).
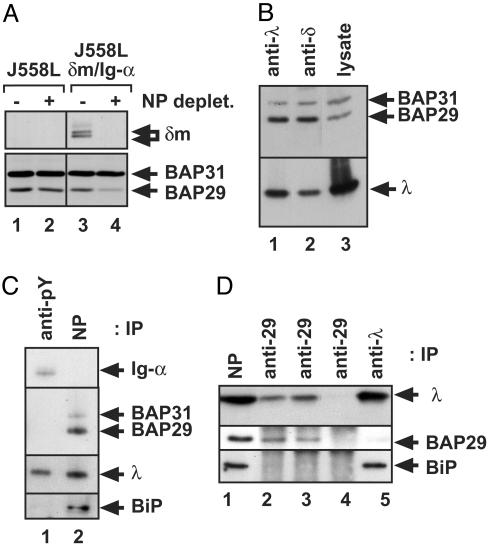
A Major Fraction of the BAP29/BAP31 Heterodimeric Pool Is Bound to mIgD. J558Lδm/Ig-α cells were used to analyze the amount of BAP29 and BAP31 associated with mIgD. Lysates were first treated with NP-Sepharose to remove all mIgD-containing complexes, and then proteins remaining in the supernatant were analyzed by SDS/PAGE and Western blotting (Fig. 3A, lanes 3 and 4). As a control, the same analysis was done with mIgD-negative J558L cells (lanes 1 and 2). Before mIgD depletion, the J558Lδm/Ig-α cell lysate contains δm, BAP29, and BAP31, whereas δm is removed on NP-Sepharose purification (Fig. 3A, lanes 3 and 4). Interestingly, the amount of BAP29, but not that of BAP31, was significantly reduced after mIgD depletion (Lower). In J558L control cells, no reduction in the amount of BAP29 or BAP31 was observed (lanes 1 and 2). Next, we purified mIgD·BAP complexes by anti-λ or anti-δ immunoprecipitations. Using SDS/PAGE and Western blotting, we analyzed the ratio of BAP29 to BAP31 in the precipitations (Fig. 3B, lanes 1 and 2), and compared it to the ratio in a total cellular lysate (lane 3). In the lysate, more BAP31 (in respect to BAP29) is found than in the purified BAP complexes. The same number of BAP29 and BAP31 bind to mIgD (20). Thus, we conclude that a cell lysate contains more BAP31 than BAP29, and unlike most BAP31, the majority of BAP29/BAP31 heterooligomers are bound to mIgD (Fig. 3A).
Fig. 3.
BAP31 occurs in a homomeric BAP31 and in a heterodimeric BAP29/BAP31 pool; only the latter is associated with mIgD. (A) Western blot analysis of δm(Upper) and BAP29/BAP31 (Lower) in cell lysates of J558L (lane 1 and 2) and J558Lδm/Ig-α cells (lane 3 and 4). The lysates were left untreated (lane 1 and 3) or incubated with NP-Sepharose to remove mIgD from the lysates (lane 2 and 4). (B) mIgD complexes were purified from J558Lδm/Ig-α lysates with anti-λ (lane 1) or anti-δ (lane 2) antisera. Proteins were separated by SDS/PAGE and detected with anti-BAP29, anti-BAP31, and anti-λ antisera. For comparison, a cellular lysate was used (lane 3). (C) Phosphotyrosine-containing proteins were purified from stimulated J558Lδm/Ig-α cells (lane 1), and mIgD complexes remaining in the supernatant were purified with NP-Sepharose (lane 2). Proteins were separated by SDS/PAGE and detected as indicated. (D) mIgD complexes were purified from J558Lδm lysates with NP-Sepharose, eluted by free hapten (lane 1), and precipitated three times with an anti-BAP29 antiserum (lanes 2, 3, and 4). Subsequently, the mIgD molecules remaining in the supernatant were purified with an anti-λ antiserum (lane 5). Proteins were separated by SDS/PAGE and detected with anti-λ, anti-BAP29, and anti-BiP antisera.
The BiP (35) binds to the first Ig domains of ER-localized soluble Ig molecules. Therefore, we investigated whether BiP also binds to ER-localized membrane-bound Ig. The same experimental approach was followed as described for Fig. 1. Indeed, BiP does not bind to the BCR complex (Fig. 3C, lane 1), but it does bind to the ER-form of mIgD (lane 2). Next, we asked whether BiP and BAP are present together in one complex with mIgD. An experimental approach as shown in Fig. 2 was applied by using the cell line J558Lδm. This cell line does not express Ig-α and thus, does neither contain a BCR nor express mIgD at the cell surface (data not shown). This finding allows us to exclusively investigate the ER-pool of mIg. The purification strategy used reveals that mIgD·BAP complexes (Fig. 3D, lanes 2 and 3) do not contain BiP, whereas (at least) some of the remaining mIgD molecules are associated with BiP (lane 5). Thus, the few BAP-associated mIgD molecules are not preferentially bound to BiP.
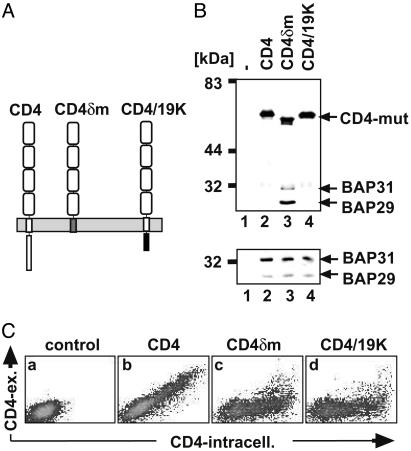
The TM Region of mIgD Is Sufficient for Both Binding to BAP29/BAP31 and Retention. Chimeric proteins based on the CD4 molecule (Fig. 4A) were used to determine whether the TM region of δm was not only necessary (22), but also sufficient for BAP-binding. In CD4δm the TM and cytoplasmic regions of CD4 were replaced by the corresponding parts of δm. CD4/19K contains the ectodomains and TM region of CD4, whereas its cytoplasmic tail is derived from E3/19K, a protein which contains a C-terminal KKXX motif and is localized in the ER. The corresponding expression vectors were transfected into D. melanogaster S2 cells, together with plasmids encoding mouse BAP29 and BAP31. Proteins were immunopurified from cell lysates with anti-CD4 antibodies and analyzed by SDS/PAGE and Western blotting (Fig. 4B Upper). As a control, total cellular lysates were analyzed for BAP expression (Lower). No BAP29/BAP31 proteins were copurified with CD4 and CD4/19K (lanes 2 and 4), although BAP29/BAP31 proteins were present in the lysates (Lower); however, BAP29/BAP31 proteins were bound to CD4δm (Fig. 4B, lane 3). This result indicates that the TM part of mIgD is necessary and sufficient for interacting with BAP29/BAP31.
Fig. 4.
Analysis of wild-type and chimeric CD4 proteins for BAP29/BAP31 binding and retention. (A) Images of CD4, CD4δm, and CD4/19K are shown. The CD4-derived sequence is white, the δm TM region is gray, and the E3/19K cytoplasmic tail is black. (B) SDS/PAGE and Western blot analysis of proteins in anti-CD4 immunoprecipitates (Upper) and total lysates (Lower) of untransfected cells (lane 1) and S2 cells cotransfected with BAP29/BAP31 and CD4 (lane 2), CD4δm (lane 3), or CD4/19K (lane 4). Western blot detection was done with anti-CD4, anti-BAP29, and anti-BAP31 antisera. (C) Untransfected (a) or CD4-transfected (b–d) S2 cells were stained with anti-CD4 antibodies (phycoerythrin) and subsequently after permeabilization intracellularly with anti-CD4 antibodies (FITC). Cells were analyzed with a flow cytometer, and the results were plotted on a double-logarithmic scale.
In the absence of Ig-α/Ig-β, most mIgD molecules are retained inside S2 cells (see below). To investigate whether the presence of the δm TM region is sufficient for retention, fluorescence-activated cell sorter (FACS) analysis was performed on S2 cells expressing different CD4 chimeric proteins (Fig. 4C). The wild-type CD4 transfectants express CD4 intracellularly and on the cell surface, indicating that CD4 is exported from the ER (Fig. 4Cb). In contrast to CD4, most CD4δm molecules are not expressed on the cell surface (Fig. 4Cc), indicating that the TM region of δm is sufficient for the retention of the chimeric molecule. CD4/19K was used as a control. As expected, the majority of this protein is expressed inside the cell, not on the surface (Fig. 4Cd).
The TM Region of mIgD Is Necessary for Retention. Because the TM region of mIgD is necessary for BAP binding (20, 22), we next investigated whether it is also necessary for retaining mIgD in the ER. To express a mIgD-like molecule in S2 cells, we generated an expression vector for a scδm molecule (Fig. 5A). In this chimera, the VH and CH1 domains of δm are replaced by a VH- and VL-containing scFv fragment derived from the NP-binding antibody B1–8. The scδm expression vector was transfected with or without BAP29 and BAP31 vectors into S2 cells. Flow cytometric analysis showed that 75% of intracellular-positive cells were surface negative, indicating that in these cells scδm is not transported to the cell surface (Fig. 5Bb). Interestingly, in 25% of the scδm-expressing cells, the molecule did appear on the surface. This result was mainly seen in cells expressing high levels of scδm, suggesting that the retention mechanism does not work when the number of scδm molecules is too large. If expression vectors for BAP29 and BAP31 were cotransfected with a plasmid coding for scδm, surface transport of scδm in cells with a high expression level was prevented (Fig. 5Bc). We also used a chimeric scδmMHCTM molecule (Fig. 5A), which does not bind to BAP proteins (data not shown). In scδmMHCTM the TM region of δm is replaced by the hydrophobic TM part of the major histocompatibility complex I (MHC I, H2-KK). FACScan analysis of transfected S2 cells showed that the amount of scδmMHCTM molecules on the cell surface is not altered after coexpression of BAP29/BAP31 (Fig. 5 Be and Bf). Thus, the effect of BAP29/BAP31 on the transport of scδm was not because of general inhibition of exocytosis.
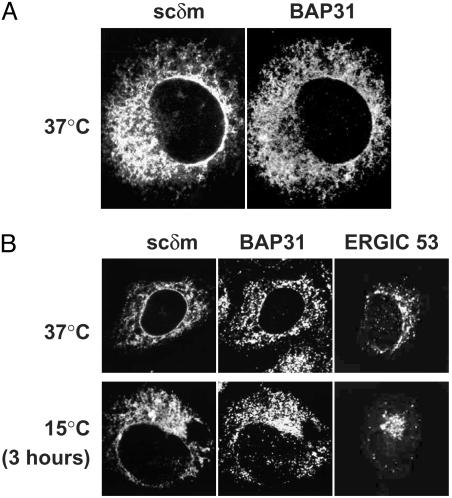
BAP31 and scδm Are Retained in the ER Without Retrieval. Cos-7 cells were transfected with an expression vector for scδm, fixed and stained simultaneously with anti-sc and anti-BAP31 antibodies, and analyzed by immunofluorescence microscopy (Fig. 6A). Both proteins colocalized and showed an ER-like distribution (including staining of the nuclear membrane). This result confirms that scδm (and possibly mIgD) are retained in the ER in the absence of Ig-α/Ig-β.
Fig. 6.
BAP31 and scδm are retained in the ER without being retrieved from a post-ER compartment. (A) Cos-7 cells were transiently transfected with an expression vector for scδm, fixed, and stained simultaneously with an anti-sc antibody (Left) and an anti-BAP31 antiserum (Right). The images were taken with a confocal microscope. (B) CHO cells transiently transfected with an expression vector for scδm were grown at 37°C(Upper), or a 3-h incubation at 15°C was included (Lower). Cells were fixed and stained with an anti-ERGIC53 antibody (Right) or simultaneously with an anti-sc antibody and an anti-BAP31 antiserum (Left and Center).
Next, the mechanism of retention of scδm, as well as of BAP31, was investigated by using a 15°C temperature shift experiment (33). At 15°C the forward transport from the ER to the intermediate compartment still takes place, whereas the retrograde transport (intermediate compartment to ER) is blocked. Thus, proteins that are retained in the ER by retrieval from post-ER compartments accumulate in the intermediate compartment. Proteins that never leave the ER do not change their localization at 15°C. We transiently transfected CHO cells with a plasmid coding for scδm and cultured them at 37°C (Fig. 6B Upper)orincludeda15°C incubation for 3 h (Fig. 6B Lower). Cells were fixed, stained with anti-sc, anti-BAP31, or anti-ERGIC53 antibodies, and analyzed by fluorescence microscopy. Neither BAP31 nor scδm accumulate outside the ER at 15°C (Fig. 6B Lower), indicating that they are truly retained in the ER. As control, ERGIC53 did accumulate in large dots, mainly in the Golgi/intermediate compartment area (Fig. 6B) as described (33).
Discussion
Affinity purification of mIgD from B cell lysates results in the copurification of the Ig-α/Ig-β as well as the BAP29/BAP31 heterodimer, which previously lead us to believe that the BAP proteins were components of the BCR complex (20). Here we show that only a minor pool of intracellular mIgD is bound to BAP proteins, whereas Ig-α/Ig-β is associated with the surface form of mIgD (Fig. 1). Thus, BAP29/BAP31 are not part of the BCR. This finding is in agreement with the localization of BAP31 to the ER (23, 25) and with a recent analysis (11) indicating that the BCR comprises only Ig-α, Ig-β, and the mIg molecule. If BAP29 and BAP31 are copurified with mIgD, these three proteins are present in an ≈1:1:1 ratio, suggesting that most mIgD is associated with the BAP proteins (20). This interpretation is not correct and we now know that this ratio is due to the copurification of two different intracellular pools of mIgD, a large pool of free mIgD molecules, and a minor pool of mIgD associated with a high-Mr BAP complex containing many BAP29/BAP31 heterodimers.
The mIg molecules are synthesized, folded, and assembled in the ER. The chaperones in the ER lumen that control folding and assembly of soluble Ig heavy and light chains are well known (36–41). Like the BAP proteins, these ER chaperones are ubiquitous, abundant, and evolutionarily strongly conserved proteins. One example is BiP, which binds to freshly synthesized soluble Ig heavy chains (38), which still have not paired to a light chain or folded correctly. Here, we show that BiP also binds to ER-localized mIg molecules, but is not part of the BCR (Fig. 3). In analogy to soluble antibodies, we suggest that also in this case BiP controls the assembly of the mIg heavy chain with the light chain. Another example is the membrane-bound, ER-localized chaperone calnexin, which is involved in the proper folding and transient binding of mIg molecules (18); however, inhibition of the association between mIg and calnexin did not result in the export of a mutant mIgD molecule (18). Thus, the retention mechanism of free mIg molecules that have not yet assembled with Ig-α/Ig-β remains enigmatic. It is known, however, that the polar amino acid residues of the mIg TM region are important for retention of free mIg (reviewed in ref. 6).
Our finding that the TM region of mIgD is sufficient to retain a chimeric CD4 molecule in the ER (Fig. 4) indicates that this region carries an autonomous ER-retention signal. Furthermore, the TM region of mIgD is necessary (22) and sufficient for BAP binding (Fig. 4). Thus, in CD4 as well as in scδm chimeric molecules, BAP binding correlates with retention of mIg.
To determine whether BAP proteins are directly involved in mIg retention, we transfected plasmids encoding these molecules into D. melanogaster S2 cells. The underlying retention machinery is evolutionarily conserved, because in scδm-transfected S2 cells with a low/moderate expression level scδm is not transported to the cell surface. D. melanogaster does indeed express one BAP homologue (S.K., unpublished results). The retention mechanism fails in cells expressing large amounts of scδm, indicating that a limiting factor is involved, which is overridden in this case. Coexpression of scδm and murine BAP29/BAP31 inhibits transport of scδm to the surface. This result directly indicates that BAP proteins play a role in retention of free mIg molecules. BAP coexpression did not inhibit ER export unspecifically, because scδmMHCTM, a chimera with a hydrophobic TM region that does not interact with BAP29/BAP31, was exported normally (Fig. 5). Thus, high-Mr BAP29/BAP31 homooligomers are part of the ER quality control system, which ensures that only correctly assembled BCRs reach the cell surface.
Antisense inhibition of BAP31 increased expression of CFTR on the cell surface (26). Similarly, increased expression of BAP31 results in a decrease of cell surface-localized CFTR, showing that BAP31 functions as a retention/retrieval protein for CFTR. Furthermore, the export of cellubrevin is facilitated by BAP31 (25). This finding seems to be in disagreement with its retention function for mIg and CFTR. However, the TM region of cellubrevin was reported to bind to BAP31 complexes, and these may have a different cellular function than BAP29/BAP31 heterodimers.
BiP and BAP bind to distinct regions of mIgD, and they do not bind simultaneously to the same mIgD molecule (Fig. 3). Thus, they might control independent processes, namely assembly with the light chain (38), and assembly with Ig-α/Ig-β, respectively. We propose that the BAP proteins mainly bind to mIgD molecules that have folded correctly at their luminal part and assembled with the light chain, but have not bound to Ig-α/Ig-β.
The BAP proteins could function as retention or as retrieval proteins for mIg. Because most ER-retained mIgD is not associated with BAP proteins (Fig. 2), the former case seems unlikely. In the latter case, BAP proteins would bind to escaped mIgD molecules in a post-ER compartment and retrieve them back to the ER by retrograde transport involving the KKXX-retrieval motif of BAP. In the ER, the mIg molecule would be released from BAP and therefore most mIg would not be BAP-associated. A 15°C temperature shift assay of transfected CHO cells shows, however, that both BAP31 and scδm cannot leave the ER (Fig. 6). Thus, it is unlikely that they are retained by retrieval. These data are in line with our earlier data (33) indicating that BAP31 is an ER-marker protein that never leaves the ER. These data also imply that BAP31 must contain an ER-localization signal that works without retrieval. Indeed, BAP31 mutants that lack the KKXX-retrieval motif are still localized to the ER (data not shown). Concerning differences between cell lines, we cannot exclude a possible retrieval in S2 or B cells.
Alternatively, BAP proteins could have an indirect effect on mIg retention by playing a role in the proper folding/assembly of mIg TM regions. The hydrophilic amino acids of the mIg TM region are involved in retention of mIg (7–10, 42–45). Hydrogen-bonding moieties have a strong energetic bias against being located in the membrane and are therefore shielded inside defined structures. Thus, partially hydrophilic TM regions are in danger of misfolding (46). BAP proteins may be chaperones helping in folding and assembly of TM segments. BAP29 and BAP31 each have three TM regions with hydrophilic and charged amino acids. Because these TM regions show the highest degree of conservation, the lipid membrane may be the environment in which they exert their function. Indeed, the BAP–mIg interaction takes place inside the lipid bilayer. In mIgD with misfolded TM regions, the TM retention signal may no longer function. This result may be the reason why S2 cells carry scδm on their surface. In this model, coexpression of murine BAP proteins prevents transport of scδm to the cell surface (Fig. 5), because they reduce the number of misfolded mIgD TM regions. The scδmMHCTM molecule is exported (Fig. 5), because it may not require BAP for proper folding because of its entirely hydrophobic TM region. Interestingly, prohibitin (BAP32) and BAP37, two TM proteins that form a high-Mr complex may possess chaperone activity for the assembly of TM protein complexes (47).
BAP31 also fulfills a second cellular function as a regulator of the apoptosis program. It binds to Bcl-2 and procaspase 8, which can cleave the cytosolic tail of BAP31 (23, 48). BAP31 might hold the proapoptotic complex in a certain conformation, such that after cleavage of its tail its structure is altered and apoptosis is induced.
Supplementary Material
Acknowledgments
We thank K. Karjalainen for the pSP6-T4, T. Nilsson for the pCMUIV-CD4/19K plasmids, J. Jongstra for the Ig-α antiserum, and G. Sher for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 388, the Leibniz Prize (to M.R.), and the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (W.W.A.S.).
Abbreviations: BCR, B cell antigen receptor; BiP, heavy chain binding protein; ER, endoplasmic reticulum; TM, transmembrane; BAP, BCR-associated protein; CFTR, cystic fibrosis TM regulator; CHO, Chinese hamster ovary; δm, Ig heavy chain isotypes of the mIgD class;λ, Ig light chain isotype of the mIgD class; NP, 4-hydroxy-3-nitrophenylacetate; mIg, membrane-bound Ig; sc, single chain.
References
- 1.Reth, M. (1992) Annu. Rev. Immunol. 10, 97–121. [DOI] [PubMed] [Google Scholar]
- 2.Reth, M., Wienands, J. & Schamel, W. W. A. (2000) Immunol. Rev. 176, 10–18. [DOI] [PubMed] [Google Scholar]
- 3.Reth, M. (1989) Nature 338, 383–384.2927501 [Google Scholar]
- 4.Wienands, J. (1999) The B Cell Antigen Receptor: Formation of Signaling Complexes and the Function of Adaptor Proteins (Springer, Berlin). [DOI] [PubMed]
- 5.Benschop, R. J. & Cambier, J. C. (1999) Curr. Opin. Immunol. 11, 143–151. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, K. S., Bäckström, B. T., Tiefenthaler, G. & Palmer, E. (1994) Semin. Immunol. 6, 393–410. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez, M., Misulovin, Z., Burkhardt, A. L., Mahajan, S., Costa, T., Bolen, J. B. & Nussenzweig, M. (1993) J. Exp. Med. 178, 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw, A. C., Mitchell, R. N., Weaver, Y. K., Campos, T. J., Abbas, A. K. & Leder, P. (1990) Cell 63, 381–392. [DOI] [PubMed] [Google Scholar]
- 9.Stevens, T. L., Blum, J. H., Foy, S. P., Matsuuchi, L. & DeFranco, A. L. (1994) J. Immunol. 152, 4397–4406. [PubMed] [Google Scholar]
- 10.Grupp, S. A., Campbell, K., Mitchell, R. N., Cambier, J. C. & Abbas, A. K. (1993) J. Biol. Chem. 268, 25776–25779. [PubMed] [Google Scholar]
- 11.Schamel, W. W. A. & Reth, M. (2000) Immunity 13, 5–14. [DOI] [PubMed] [Google Scholar]
- 12.Hombach, J., Leclercq, L., Radbruch, A., Rajewsky, K. & Reth, M. (1988) EMBO J. 7, 3451–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkitaraman, A. R., Williams, G. T., Dariavach, P. & Neuberger, M. S. (1991) Nature 352, 777–781. [DOI] [PubMed] [Google Scholar]
- 14.Weiser, P., Riesterer, C. & Reth, M. (1994) Eur. J. Immunol. 24, 665–671. [DOI] [PubMed] [Google Scholar]
- 15.Wienands, J., Hombach, J., Radbruch, A., Riesterer, C. & Reth, M. (1990) EMBO J. 9, 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wienands, J. & Reth, M. (1991) Eur. J. Immunol. 21, 2373–2378. [DOI] [PubMed] [Google Scholar]
- 17.Williams, G. T., Dariavach, P., Venkitaraman, A. R., Gilmore, D. J. & Neuberger, M. S. (1993) Mol. Immunol. 30, 1427–1432. [DOI] [PubMed] [Google Scholar]
- 18.Wu, Y., Pun, C. & Hozumi, N. (1997) J. Immunol. 158, 2762–2770. [PubMed] [Google Scholar]
- 19.Matsuuchi, L., Gold, M. R., Travis, A., Grosschedl, R., DeFranco, A. L. & Kelly, R. B. (1992) Proc. Natl. Acad. Sci. USA 89, 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, K.-M., Adachi, T., Nielsen, P. J., Terashima, M., Lamers, M. C., Köhler, G. & Reth, M. (1994) EMBO J. 13, 3793–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terashima, M., Kim, K.-M., Adachi, T., Nielsen, P. J., Reth, M., Köhler, G. & Lamers, M. C. (1994) EMBO J. 13, 3782–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adachi, T., Schamel, W. W. A., Kim, K. M., Watanabe, T., Becker, B., Nielsen, P. J. & Reth, M. (1996) EMBO J. 15, 1534–1541. [PMC free article] [PubMed] [Google Scholar]
- 23.Ng, F. W., Nguyen, M., Kwan, T., Branton, P. E., Nicholson, D. W., Cromlish, J. A. & Shore, G. C. (1997) J. Cell Biol. 139, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teasdale, R. D. & Jackson, M. R. (1996) Annu. Rev. Cell Dev. Biol. 12, 27–54. [DOI] [PubMed] [Google Scholar]
- 25.Annaert, W. G., Becker, B., Kistner, U., Reth, M. & Jahn, R. (1997) J. Cell Biol. 139, 1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert, G., Becker, B., Schreiber, R., Boucherot, A., Reth, M. & Kunzelmann, K. (2001) J. Biol. Chem. 276, 20340–20345. [DOI] [PubMed] [Google Scholar]
- 27.Bunch, T. A., Grinblat, Y. & Goldstein, L. S. (1988) Nucleic Acids Res. 16, 1043–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolli, V., Gallwitz, M., Wossning, T., Flemming, A., Schamel, W., Zürn, C. & Reth, M. (2002) Mol. Cell 10, 1057–1069. [DOI] [PubMed] [Google Scholar]
- 29.Kappeler, F., Klopfenstein, D. R., Foguet, M., Paccaud, J. P. & Hauri, H. P. (1997) J. Biol. Chem. 272, 31801–31808. [DOI] [PubMed] [Google Scholar]
- 30.Reth, M., Imanishi-Kari, T. & Rajewsky, K. (1979) Eur. J. Immunol. 9, 1004–1013. [DOI] [PubMed] [Google Scholar]
- 31.Wienands, J., Larbolette, O. & Reth, M. (1996) Proc. Natl. Acad. Sci. USA 93, 7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flaswinkel, H. & Reth, M. (1994) EMBO J. 13, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klumperman, J., Schweizer, A., Clausen, H., Tang, B. L., Hong, W., Oorschot, V. & Hauri, H.-P. (1998) J. Cell Sci. 111, 3411–3425. [DOI] [PubMed] [Google Scholar]
- 34.Schamel, W. W. A. & Reth, M. (2000) Mol. Immunol. 37, 253–259. [DOI] [PubMed] [Google Scholar]
- 35.Haas, I. G. & Wabl, M. (1983) Nature 306, 387–389. [DOI] [PubMed] [Google Scholar]
- 36.Melnick, J. & Argon, Y. (1995) Immunol. Today 16, 243–250. [DOI] [PubMed] [Google Scholar]
- 37.Kaloff, C. R. & Haas, I. G. (1995) Immunity 2, 629–637. [DOI] [PubMed] [Google Scholar]
- 38.Bole, D. G., Hendershot, L. M. & Kearney, J. F. (1986) J. Cell Biol. 102, 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O`Hare, T., Wiens, G. D., Whitcomb, E. A., Enns, C. A. & Rittenberg, M. B. (1999) J. Immunol. 163, 11–14. [PubMed] [Google Scholar]
- 40.Ho, S. C., Chaudhuri, S., Bachhawat, A., McDonald, K. & Pillai, S. (2000) J. Immunol. 164, 4713–4719. [DOI] [PubMed] [Google Scholar]
- 41.Hendershot, L. M. (1990) J. Cell Biol. 111, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams, G. T., Venkitaraman, A. R., Gilmore, D. J. & Neuberger, M. S. (1990) J. Exp. Med. 171, 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blum, J. H., Stevens, T. L. & DeFranco, A. L. (1993) J. Biol. Chem. 268, 27236–27245. [PubMed] [Google Scholar]
- 44.Patel, K. J. & Neuberger, M. S. (1993) Cell 74, 939–946. [DOI] [PubMed] [Google Scholar]
- 45.Cherayil, B. J., MacDonald, K., Waneck, G. L. & Pillai, S. (1993) J. Immunol. 151, 11–19. [PubMed] [Google Scholar]
- 46.Sanders, C. R. & Nagy, J. K. (2000) Curr. Opin. Struct. Biol. 10, 438–442. [DOI] [PubMed] [Google Scholar]
- 47.Nijtmans, L. G., de Jong, L., Artal Sanz, M., Coates, P. J., Berden, J. A., Willem Back, J., Muijsers, A. O., van der Spek, H. & Grivell, L. A. (2000) EMBO J. 19, 2444–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breckenridge, D., Nguyen, M., Kuppig, S., Reth, M. & Shore, G. (2002) Proc. Natl. Acad. Sci. USA 99, 4331–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.