Abstract
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) are central components of the machinery mediating membrane fusion in all eukaryotic cells. Sequence analysis of the yeast genome revealed a previously uncharacterized SNARE, SNARE-like tail-anchored protein 1 (Slt1). Slt1 is an essential protein localized in the endoplasmic reticulum (ER). It forms a SNARE complex with Sec22 and the ER syntaxin Ufe1. Down-regulation of Slt1 levels leads to improper secretion of proteins normally resident in the ER. We suggest that Slt1 is a component of the SNAREpin required for retrograde traffic to the ER. Based on the previously reported association with Ufe1 and Sec22, Sec20 likely contributes the fourth SNARE to the SNAREpin.
Keywords: membrane fusion, Slt1, secretion, tail-anchored protein
Fusion of biological membranes is a fundamental process governing transport of cargo molecules throughout the secretory and endocytic pathway. The central components driving intracellular membrane fusion are compartment-specific proteins called v- and t-SNAREs [(SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor localized on vesicles (v) or target (t) membranes)] that assemble into cognate pairs between two lipid bilayers forming SNAREpins (trans v-/t-SNARE complexes) (1, 2). Each SNAREpin is composed of a bundle of four α-helices, three derived from the t-SNARE (a syntaxin heavy chain and two SNARE light chains) and the fourth from the cognate v-SNARE (3, 4). SNAREpin assembly likely starts at the amino-terminal, membrane-distal end, proceeds in a membrane-proximal direction, bringing the two lipid bilayers in close apposition, and finally culminates in membrane fusion (2, 5–7). Fully assembled cis v-/t-SNARE complexes (all SNAREs residing in the same lipid bilayer) then bind N-ethylmaleimide-sensitive factor attachment protein α (α-SNAP) and NSF. Subsequent ATP-hydrolysis by NSF disassembles the complex and recycles individual SNAREs for another round of transport (8).
The initial membrane trafficking event in the secretory pathway requires the fusion of endoplasmic reticulum (ER)-derived COPII-coated, anterograde-directed vesicles with compartments on the cis side of the Golgi. SNAREs required for this anterograde transport have been identified in yeast and include Sed5, Bos1, Sec22, and Bet1 (9). Although all four SNAREs are present both on the vesicle and cis-Golgi membranes, they seem to be required in an asymmetric fashion, either on the vesicle or target membrane (10). In vitro fusion assays have demonstrated that SNAREs indeed function in a topologically restricted manner. Liposomes containing the t-SNARE heavy chain (syntaxin) Sed5 and the light chains Bos1 and Sec22 specifically fuse with v-SNARE liposomes containing Bet1; other topological combinations of these SNAREs are not fusogenic (11).
To prevent a prominent net membrane efflux from the ER, to recycle the transport machinery, and to retrieve resident proteins that escaped the ER, retrograde-directed vesicles budding at the cis face of the Golgi and throughout the Golgi stack balance the overall membrane flow. Candidate proteins involved in the fusion of retrograde-directed vesicles with the ER have been identified in yeast. Genetic and biochemical evidence implicates that Sec22 functions as a retrograde SNARE (in addition to its role as a cis-Golgi t-SNARE light chain) (12, 13). Indeed, the sec22-3 mutant is characterized by an increased secretion of the ER-resident protein Kar2 (BIP), suggesting a defect in retrograde transport (14). The syntaxin Ufe1 is localized to the ER and genetically and biochemically interacts with Sec22, indicating a t-SNARE function (13, 14). Two additional proteins, Sec20 and Tip20, are found in a complex with Ufe1 (14–16). Although Sec20, based on its sequence, has not been classified as a SNARE, it contains a coil-coiled motif adjacent to the transmembrane domain. By lowering the stringency for a sequence alignment, it can be classified as a SNARE (Fig. 1B). In contrast, Tip20 is a peripheral protein associated with Sec20 and could play a regulatory role. All known functional SNAREpins are assembled from four SNAREs, yet there are only three known candidates for the subunits of the retrograde SNAREpin: Ufe1, Sec22, and Sec20.
Fig. 1.
(A) Amino acid sequence of Slt1. The SNARE motif is boxed in gray and the transmembrane domain is in boldface type. (B) Alignment of Slt1 and Sec20 with SNARE motif classes in yeast. Numbers denote residues in helix–helix contacts, the residue in the “0 layer” is boxed in black, and hydrophobic residues in the helix–helix contacts positions are shaded (37–39).
To identify the missing fourth SNARE, which functions in the retrograde pathway from the Golgi to the ER, we undertook a comprehensive sequence analysis of the Saccharomyces cerevisiae genome. Our analysis revealed a SNARE protein termed SNARE-like tail-anchored protein 1 (Slt1). Slt1 proved to be an essential, type II integral membrane protein localized to the ER. Slt1 is associated with the retrograde SNAREs Ufe1 and Sec22. It is required for the retention of ER resident proteins. Therefore, Slt1 is a component of the retrograde transport machinery, likely to be the fourth subunit of the retrograde SNAREpin.
Materials and Methods
Sequence Analysis. The S. cerevisiae genome was scanned by using tmpred (www.ch.embnet.org/software/TMPRED_form.html) to identify those ORFs that might encode proteins with a single transmembrane segment. Constraints were imposed on the initial data: (i) the hydrophobic segment should be positioned such that less than one-third of the protein would be located as a luminal (trans) domain; (ii) to be rid of sequences encoding GPI-anchored proteins and type I membrane proteins, ORFs were screened for the presence of a signal sequence by using signalp (17); (iii) to remove fortuitous, small, and nonfunctional ORFs, transcriptional databases were analyzed by using each sequence as a query.
Plasmids, Yeast Strain, and Media. The plasmid coding for recombinant GST-α-SNAP has been described (18). Oligonucleotide primers were used to attach a signal sequence and the HDEL retention sequence to GFP-S65T, equivalent to the construct ER-GFP (19), but for the expression in yeast the plasmid chosen has the MET25 promoter (20) and the carboxyl-terminal sequence of the modified GFP-S65T is YKSGLRSRAHDEL. DNA fragments corresponding to the Slt1 ORF (YGL098w) were amplified by PCR using primers that generated one inframe restriction site immediately preceding the start codon, and another following the stop codon (oligonucleotide sequences available on request). PCR products were subcloned behind GFP-S65T under the control of the MET25 promoter (20) or behind a sequence encoding a triple hemagglutinin (HA) epitope under the control of the GAL1 promoter in the plasmid pYX213. The wild-type strain BY4742 (MATα, his3Δ, leu2Δ, lys2Δ, ura3Δ) and the SLT1/Δslt1 strain 24465 (American Type Culture Collection) were used as a basis for the Kar2 secretion assay, whereas the temperature-sensitive strain sec18-1 (MATα, sec18-1, his4-619, ura3-52) (21) was used for HA-Slt1 pull-down assays. The SLT1/Δslt1 strain was transformed with these plasmids, sporulated and dissected to derive haploid strains expressing only the epitope-tagged Slt1.
Na2CO3 Extraction and Protease Sensitivity. Microsomal membrane fractions were prepared by differential centrifugation. One hundred OD600 units of cells were suspended in lysis buffer (250 mM sorbitol/150 mM potassium acetate/5 mM magnesium acetate/0.5 mM PMSF/1.2 μg/ml leupeptin/0.75 μg/ml antipain/0.25 μg/ml chymostatin/1 μg/ml pepstatin/50 mM Hepes, pH 6.8) and disrupted by two 2-min bursts in a minibeadbeater-8 (Biospec Products, Bartlesville, OK) with silica/zirconia beads. Cell debris was removed by centrifugation at 500 × g for 5 min. A crude membrane fraction was collected by centrifugation at 16,000 × g for 10 min. Membranes were extracted by resuspension in either 1% Triton X-100 or 100 mM Na2CO3 and incubation for 30 min on ice with intermittent vortexing (22). Soluble and insoluble proteins were separated by centrifugation at 100,000 × g in a Beckman Airfuge. Trypsin treatments were performed as described (23).
HA Pull-Down Assays. Detergent extracts of sec18-1 were prepared as described (24). Briefly, 20,000 OD units of sec18-1 cells were spheroplasted at 25°C and subsequently incubated for 1 h at 37°C. After incubation, cells were isolated by centrifugation and disrupted with glass beads in 50–100 ml of ice-cold lysis buffer (50 mM Hepes, pH 7.4/0.1 M KCl/1% Triton X-100/1% glycerol/1 mM EDTA/1 mM DTT/protease inhibitors: 1 μg/ml leupeptin/2 μg/ml antipain/20 μg/ml trypsin inhibitor/10 μg/ml benzamidine/5 μg/ml pefabloc SC/2 μg/ml aprotinin/4 μg/ml chymostatin/2 μg/ml pepstatin). Unbroken cells were removed by centrifugation for 1 h at 100,000 × g. The protein concentration of the supernatant was adjusted to 2 mg/ml with lysis buffer. A 50-ml extract was incubated overnight at 4°C with 2 ml of glutathione beads containing 500–1,000 μg of GST-α-SNAP. In control experiments, the extract was incubated with GST-glutathione beads. Beads were washed with lysis buffer, and assembled SNARE complexes were eluted with two 5-ml portions of lysis buffer containing 1 M NaCl. The eluate was adjusted to 0.15 M NaCl with lysis buffer and incubated overnight at 4°C with 1.8 ml of high affinity anti-HA Matrix (Roche Diagnostics). After incubation, beads were washed with lysis buffer and then with lysis buffer containing 0.3 M NaCl. Protein complexes were eluted with 1 ml of 0.2 M glycine (pH 2.8) and precipitated by using 5% (vol/vol) trichloroacetic acid. Precipitated proteins were subjected to Western blot analysis using the ECL Plus Western Blotting Detection System (Amersham Pharmacia). Typically, 10% of the eluted material was loaded in each lane. Where indicated, yeast lysate was directly incubated with anti-HA affinity beads. Polyclonal antibodies to Sed5, Bos1, Sec22, Bet1 (24), Gos1 (25, 26), and Sft1 (27) have been described. Polyclonal antibodies to Vti1 and Snc1 were generated in rabbit by using the corresponding His-tagged proteins. Monoclonal anti-HA peroxidase antibodies (Roche Molecular Biochemicals) were used for the detection of HA-Slt1. The anti-Ufe1 antibody was a generous gift of Hugh Pelham (Medical Research Council Laborator y of Molecular Biology, Cambridge, U.K.).
COPII Budding Assay. In vitro budding reactions with purified COPII proteins were performed as described (28). Briefly, microsomes were prepared from cells expressing GFP-Slt1 and stripped of endogenous coat proteins by washing with 2.5 M urea in B88 [20 mM Hepes, pH 6.8/250 mM sorbitol/150 mM KOAc/5 mM Mg(OAC)2]. Purified COPII proteins Sar-1, Sec23/24, and Sec13/31 were added in a ratio of 10:10:20 mg/ml to 300 mg of washed microsomes in the presence or absence of 0.1 mM guanylyl imidodiphosphate (GMP-PNP) and incubated for 30 min at room temperature. Of the total reaction, 10% was removed and diluted in B88. Vesicles in the remaining sample were purified by differential centrifugation, first at 17,610 × g for 15 min, using a Tomy (Tokyo) MTX-150 swing-out centrifuge, to sediment donor ER membranes, then at 125,000 × g for 20 min, using a Beckman bench-top ultracentrifuge with a TL100.3 rotor, to pellet vesicles. The diluted total membranes were collected in parallel. Membrane pellets were solubilized in SDS sample buffer and analyzed by Western blot.
Miscellaneous. Fluorescence images were captured by using a Bio-Rad MRC1024 Confocal Scanning Laser microscope mounted on a Zeiss Axioscop. Cells were grown to mid log phase at 30°C in selective media before analysis.
Results
Because the vast majority of SNARE proteins are type II membrane proteins, we analyzed all ORFs in the yeast genome that encode a single hydrophobic segment and could function as a carboxyl-terminal membrane anchor. In a total of 55 proteins, a SNARE protein termed Slt1 was identified (YGL098w). Slt1 is a 245-aa protein and contains an amino-terminal domain followed by a predicted carboxyl-terminal transmembrane domain (residues 219–239) (Fig. 1A). Directly preceding the transmembrane domain is a sequence that has a significant propensity to form coiled-coils and shows the characteristics of a SNARE motif. Sequence alignment classifies Slt1 as a Qc/SNAP Cs-SNARE (Fig. 1B; ref. 39). Although the overall sequence similarity between Slt1 and the other SNAREs is low, the sequence of Slt1 is most homologous to the Golgi SNARE Sft1, both containing an aspartate at the “0 layer” of the SNARE motif (Fig. 1B).
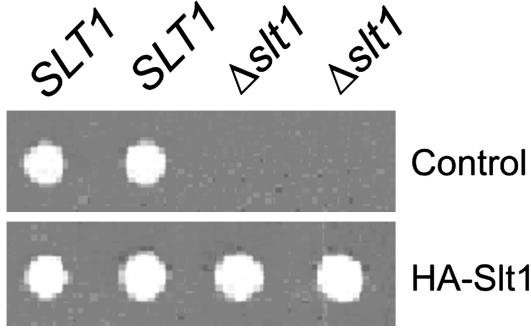
Slt1 is an essential protein: when one copy of the SLT1 gene was deleted in diploid yeast cells and the SLT1/Δslt1 cells induced to undergo meiosis, the haploid Δslt1 cells failed to grow (Fig. 2). Transformation of the cells with a plasmid encoding GFP protein fused to the amino terminus of Slt1 (data not shown) or a HA-tagged Slt1 (Fig. 2) rescues the cells.
Fig. 2.
Slt1 is an essential protein. SLT1/Δslt1 yeast cells were transformed with a plasmid encoding HA-tagged Slt1 or a control plasmid. The cells were transferred to sporulation medium to induce meiosis, and the resulting spores were dissected onto plates of rich medium. Colonies were viewed after 3 days' growth at 30°C.
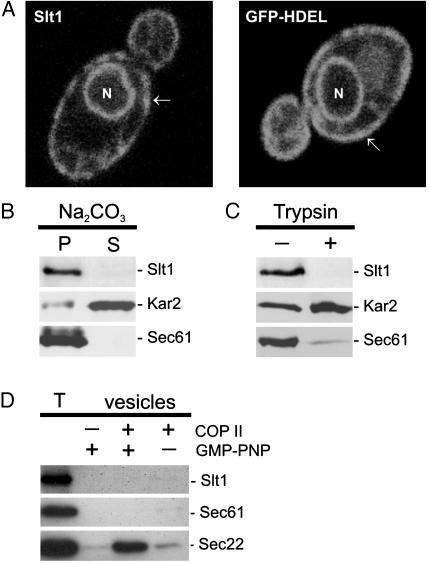
Because the tagged Slt1 proteins are functional, they were used to determine the intracellular localization of Slt1. Both GFP-Slt1 and HA-Slt1 label the nuclear membrane and membrane structures underlying the plasma membrane, which are both characteristic for the typical ER localization in yeast (Fig. 3A and data not shown). A GFP fusion protein containing the ER-retrieval sequence HDEL, which is an established ER marker, shows a similar staining pattern (Fig. 3A; ref. 19). To establish that Slt1 is indeed a type II membrane protein, crude yeast membrane preparations were treated with either sodium carbonate or low amounts of trypsin. As expected, Slt1, like the ER marker Sec61, is not extracted at pH 11.5 and therefore behaves as an integral membrane protein (Fig. 3B). Slt1 is protease-sensitive, which clearly demonstrates that the majority of the protein (the amino terminus) is exposed in the cytoplasm (Fig. 3C). In contrast, the soluble, luminal ER marker Kar2 is protected from the protease digestion but sensitive to sodium carbonate extraction. To analyze whether Slt1, like Sec22, enters COPII-coated vesicles budding at the ER, isolated microsomes were incubated with purified COPII components and budding of vesicles was monitored (28). In contrast to Sec22, but like Ufe1, Slt1 does not enter in significant amounts COPII vesicles and behaves as a permanent ER resident (Fig. 3D; ref. 29).
Fig. 3.
Slt1 is an integral membrane protein permanently localized in the ER. (A) Slt1 is localized to the ER. Yeast cells expressing GFP-Slt1 or GFP-HDEL (ER retention signal) were analyzed by confocal fluorescence microscopy. Both fusions target GFP to the ER (arrows, peripheral membrane; N, nucleus). (B) Slt1 is an integral membrane protein. A crude membrane fraction was isolated from yeast cells expressing GFP-Slt1, treated with 0.1M Na2CO3, and centrifuged to separate solubilized proteins (S) from insoluble material (P). (C) Slt1 is a type II membrane protein. The membrane fraction was incubated in the presence (+) or absence (–)of1.5 μg of trypsin. (D) Slt1 is not packaged into COPII vesicles. Isolated microsomes were incubated with purified Sar-1, Sec23/24, Sec13/31, and GMP-PNP as indicated. COP II vesicles were isolated as described in Material and Methods. T shows 10% of the microsomes in the budding reaction. Proteins were separated by SDS/PAGE and detected by Western blot analyses.
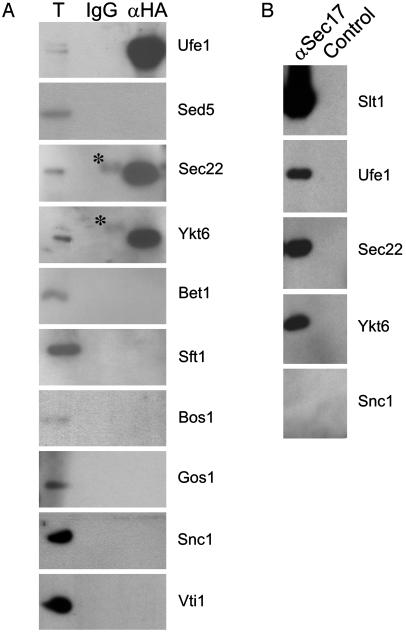
To test whether Slt1 forms a specific complex with SNAREs required for the retrograde transport to the ER, we prepared detergent extract from a temperature-sensitive sec18-1 (yeast NSF) strain expressing HA-Slt1. This yeast strain accumulates SNARE complexes at nonpermissive temperature (24). HA-Slt1 was isolated with an anti-HA antibody, and coprecipitated proteins were detected by Western blot analysis. HA-Slt1 is found in a complex with the ER syntaxin Ufe1 but not with the Golgi syntaxin Sed5. It also interacts with Sec22 and Ykt6, but not with any other SNAREs tested (Fig. 4A), demonstrating the specificity of the interaction. To further confirm the presence of Slt1 in assembled v-/t-SNARE complexes, we made use of an α-SNAP column, which binds with high affinity assembled v-/t-SNARE complexes, but not at all, or only weakly, monomeric SNAREs (30). SNARE complexes were eluted from the α-SNAP affinity column, and HA-Slt1-containing complexes were further purified with anti-HA antibodies. Western blot analysis confirmed the presence of Ufe1, Sec22, and Ykt6 in the Slt1 precipitates (Fig. 4B).
Fig. 4.
Slt1 forms a complex with SNAREs implicated in retrograde transport to the ER. (A) Detergent extracts of sec18-1 cells, which had been transformed with HA-Slt1, were incubated with an anti-HA affinity column, and coprecipitated proteins were detected by Western blot analysis. Asterisks indicate nonspecific IgG cross-reactivity. (B) Detergent extracts of sec18-1 cells, which had been transformed with HA-Slt1, were incubated with a GST-α-SNAP affinity column. Assembled v-/t-SNARE complexes were eluted with 1 M NaCl and immunoprecipitated with an anti-HA antibody, and Slt1-associated proteins were detected by Western blot analysis.
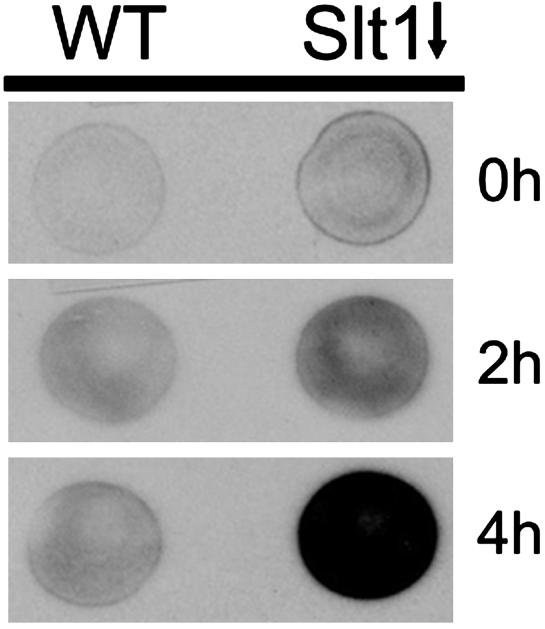
If Slt1 is required for retrograde transport, its inactivation should result in the secretion of ER residents, which are characterized by ER retrieval signals. Such a phenotype was observed when Ufe1, Sec22, and Sec20, all implicated in retrograde transport, had been inactivated (12–14). Therefore, a yeast strain that allowed the controlled expression of the SLT1 gene was engineered, and the secretion of Kar2 (the prototype protein containing an ER retrieval signal) was monitored (31). After 4 h of Slt1 repression, a significant amount of Kar2 was secreted into the extracellular medium (Fig. 5). This finding demonstrates that Slt1 is a SNARE required for retrograde transport to the ER.
Fig. 5.
Inactivation of Slt1 results in the secretion of ER residents. Wild-type cells or Δslt1 cells expressing SLT1 under control of the GAL promoter were grown to log phase in rich medium with galactose as a carbon source (YPGal). To repress expression of SLT1, cells were spotted onto glucose plates (YPAD) and incubated at 30°C for 0, 2, or 4 h, then overlaid with nitrocellulose filters and allowed to grow at 30°C for an additional hour. Kar2 that had been secreted and bound to the nitrocellulose was detected by immunoblotting with an anti-Kar2 antibody.
Discussion
The integrity of the secretory pathway relies on the balanced interplay between anterograde and retrograde transport. Whereas the anterograde pathway between the ER and the Golgi is relatively well defined, very little is known about the retrograde transport machinery functioning at the ER. Thus, the characterization of components mediating retrograde transport, in particular the identification of the corresponding SNAREpin that contributes targeting specificity and confers membrane fusion, will be of great importance.
Our bioinformatic analysis revealed a previously uncharacterized SNARE protein, Slt1, and also uncovered the recently identified Syn8, indicating that (at least in terms of classical type II membrane proteins) we are approaching complete set of SNAREs in the yeast genome (this work and ref. 32). Thus, the total number of SNAREs in yeast has reached 24 (counting SNARE isoforms, such as Snc1/2 and Sso1/2, and considering Sec20 as a putative SNARE). Slt1 shows all characteristics of a SNARE required for retrograde transport to the ER. First, Slt1 is localized to the ER; second, it forms a specific complex with Ufe1 and Sec22, which have been implicated in the retrograde pathway in yeast; and finally, it is functionally required for the retention of the ER-resident protein Kar2 (refs. 14, 33, and 34 and this work).
Our immunoprecipitation indicates that Ufe1, Slt1, and Sec22 form a SNARE complex. Previous results of Pelham and coworkers (14) already showed the existence of a complex between Ufe1, Sec22, and Sec20, suggesting that the ER SNAREpin consists of Ufe1, Slt1, Sec20, and Sec22. Because of a lack of anti-Sec20 antibodies, we were unable to provide direct evidence for the existence of this tetrameric complex. Thus, we cannot completely rule out the possibility that distinct SNARE complexes containing either Slt1 or Sec20 might exist. The fact that both Ufe1 and Slt1 are not packed into COPII-coated vesicles suggests that these proteins are part of the t-SNARE with Sec20 as the putative additional t-SNARE light chain and Sec22 as the cognate v-SNARE.
The presence of Ykt6 in the Slt1 immunoprecipitates was unexpected. We also found a small amount of endogenous Ykt6 in precipitates with antibodies against Ufe1 (data not shown). In contrast, a myc-tagged Ykt6 expressed in yeast seems not to associate with Ufe1 (14); the reason for this difference remains to be resolved. Interestingly, overexpression of Ykt6 can rescue Sec22-deficient yeast (35). Given the fact that Sec22 does not interact with Ykt6 in vivo, and that Ykt6 and Sec22 belong to the same SNARE class (R-SNARE), it is unlikely that these two SNAREs functionally participate in membrane fusion within the same SNAREpin (27). Therefore, we favor a model where Sec22 and Ykt6 are part of two distinct SNAREpins. The observation that overexpression of Ykt6 can replace Sec22 in vivo would be consistent with the above model and the partial functional redundancy of Sec22, and could explain why Sec22 is not an essential protein (35). Further, Ykt6 has also been found to associate with vacuolar SNAREs, and in this context, it has been shown that Ykt6 and the v-SNARE Nyv1 compete for binding to the vacuolar t-SNARE, again suggesting the presence of two distinct SNARE complexes (4, 36). However, the exact role of Ykt6 remains to be determined, and a regulatory function (in contrast to a direct fusogenic role) cannot be excluded.
In summary, we identified a SNARE that is involved in retrograde transport to the ER. The putative ER SNAREpin likely consists of the t-SNARE Ufe1/Slt1, Sec20, and the v-SNARE Sec22. Ykt6 may be part of the second ER SNARE-pin or play a regulatory function. To unambiguously reveal the exact role of these proteins, a functional reconstitution in an in vitro fusion assay will be required.
Acknowledgments
We thank Nia Bryant, Hugh Pelham, Jeff Schatz, and Randy Schekman for plasmids, antisera, and COPII budding components, and Binks Wattenberg for critical discussions throughout the course of the project. This work was supported by a fellowship from the Swiss National Science Foundation (to L.B.) and a grant from the Australian Research Council (to T.L.).
Abbreviations: ER, endoplasmic reticulum; α-SNAP, N-ethylmaleimide-sensitive factor attachment protein α; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
References
- 1.Söllner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. & Rothman, J. E. (1993) Nature 362, 318–324. [DOI] [PubMed] [Google Scholar]
- 2.Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T. H. & Rothman, J. E. (1998) Cell 92, 759–772. [DOI] [PubMed] [Google Scholar]
- 3.Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. (1998) Nature 395, 347–353. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda, R., McNew, J. A., Weber, T., Parlati, F., Engel, T., Nickel, W., Rothman, J. E. & Söllner, T. H. (2000) Nature 407, 198–202. [DOI] [PubMed] [Google Scholar]
- 5.Melia, T. J., Weber, T., McNew, J. A., Fisher, L. E., Johnston, R. J., Parlati, F., Mahal, L. K., Söllner, T. H. & Rothman, J. E. (2002) J. Cell Biol. 158, 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua, S. Y. & Charlton, M. P. (1999) Nat. Neurosci. 2, 1078–1083. [DOI] [PubMed] [Google Scholar]
- 7.Xu, T., Rammner, B., Margittai, M., Artalejo, A. R., Neher, E. & Jahn, R. (1999) Cell 99, 713–722. [DOI] [PubMed] [Google Scholar]
- 8.Söllner, T., Bennett, M. K., Whiteheart, S. W., Scheller, R. H. & Rothman, J. E. (1993) Cell 75, 409–418. [DOI] [PubMed] [Google Scholar]
- 9.Pelham, H. R. (1999) Exp. Cell Res. 247, 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Cao, X. & Barlowe, C. (2000) J. Cell Biol. 149, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parlati, F., McNew, J. A., Fukuda, R., Miller, R., Söllner, T. H. & Rothman, J. E. (2000) Nature 407, 194–198. [DOI] [PubMed] [Google Scholar]
- 12.Semenza, J. C., Hardwick, K. G., Dean, N. & Pelham, H. R. (1990) Cell 61, 1349–1357. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, M. J. & Pelham, H. R. (1996) Cell 85, 205–215. [DOI] [PubMed] [Google Scholar]
- 14.Lewis, M. J., Rayner, J. C. & Pelham, H. R. (1997) EMBO J. 16, 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweet, D. J. & Pelham, H. R. (1993) EMBO J. 12, 2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweet, D. J. & Pelham, H. R. (1992) EMBO J. 11, 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. (1997) Protein Eng. 10, 1–6. [DOI] [PubMed] [Google Scholar]
- 18.Paek, I., Orci, L., Ravazzola, M., Erdjument-Bromage, H., Amherdt, M., Tempst, P., Sollner, T. H. & Rothman, J. E. (1997) J. Cell Biol. 137, 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roderick, H. L., Campbell, A. K. & Llewellyn, D. H. (1997) FEBS Lett. 405, 181–185. [DOI] [PubMed] [Google Scholar]
- 20.George, R., Beddoe, T., Landl, K. & Lithgow, T. (1998) Proc. Natl. Acad. Sci. USA 95, 2296–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser, C. A. & Schekman, R. (1990) Cell 61, 723–733. [DOI] [PubMed] [Google Scholar]
- 22.Fujiki, Y., Hubbard, A. L., Fowler, S. & Lazarow, P. B. (1982) J. Cell Biol. 93, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beilharz, T., Suzuki, C. K. & Lithgow, T. (1998) J. Biol. Chem. 273, 35268–35272. [DOI] [PubMed] [Google Scholar]
- 24.Søgaard, M., Tani, K., Ye, R. R., Geromanos, S., Tempst, P., Kirchhausen, T., Rothman, J. E. & Sollner, T. (1994) Cell 78, 937–948. [DOI] [PubMed] [Google Scholar]
- 25.McNew, J. A., Coe, J. G., Søgaard, M., Zemelman, B. V., Wimmer, C., Hong, W. & Söllner, T. H. (1998) FEBS Lett. 435, 89–95. [DOI] [PubMed] [Google Scholar]
- 26.McNew, J. A., Søgaard, M., Lampen, N. M., Machida, S., Ye, R. R., Lacomis, L., Tempst, P., Rothman, J. E. & Söllner, T. H. (1997) J. Biol. Chem. 272, 17776–17783. [DOI] [PubMed] [Google Scholar]
- 27.Parlati, F., Varlamov, O., Paz, K., McNew, J. A., Hurtado, D., Söllner, T. H. & Rothman, J. E. (2002) Proc. Natl. Acad. Sci. USA 99, 5424–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M. F., Ravazzola, M., Amherdt, M. & Schekman, R. (1994) Cell 77, 895–907. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka, K., Morimitsu, Y., Uchida, K. & Schekman, R. (1998) Mol. Cell 2, 703–708. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi, T., Yamasaki, S., Nauenburg, S., Binz, T. & Niemann, H. (1995) EMBO J. 14, 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munro, S. & Pelham, H. R. (1987) Cell 48, 899–907. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, M. J. & Pelham, H. R. (2002) Traffic 3, 922–929. [DOI] [PubMed] [Google Scholar]
- 33.Ballensiefen, W., Ossipov, D. & Schmitt, H. D. (1998) J. Cell Sci. 111, 1507–1520. [DOI] [PubMed] [Google Scholar]
- 34.Spang, A. & Schekman, R. (1998) J. Cell Biol. 143, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Y. & Barlowe, C. (2002) Mol. Biol. Cell 13, 3314–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungermann, C., von Mollard, G. F., Jensen, O. N., Margolis, N., Stevens, T. H. & Wickner, W. (1999) J. Cell Biol. 145, 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weimbs, T., Low, S. H., Chapin, S. J., Mostov, K. E., Bucher, P. & Hofmann, K. (1997) Proc. Natl. Acad. Sci. USA 94, 3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fasshauer, D., Sutton, R. B., Brunger, A. T. & Jahn, R. (1998) Proc. Natl. Acad. Sci. USA 95, 15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bock, J. B., Matern, H. T., Peden, A. A. & Scheller, R. H. (2001) Nature 409, 839–841. [DOI] [PubMed] [Google Scholar]