Abstract
Concentrations of biodiversity, or hotspots, represent conservation priorities in terrestrial ecosystems but remain largely unexplored in marine habitats. In the open ocean, many large predators such as tunas, sharks, billfishes, and sea turtles are of current conservation concern because of their vulnerability to overfishing and ecosystem role. Here we use scientific-observer records from pelagic longline fisheries in the Atlantic and Pacific Oceans to show that oceanic predators concentrate in distinct diversity hotspots. Predator diversity consistently peaks at intermediate latitudes (20–30° N and S), where tropical and temperate species ranges overlap. Individual hotspots are found close to prominent habitat features such as reefs, shelf breaks, or seamounts and often coincide with zooplankton and coral reef hotspots. Closed-area models in the northwest Atlantic predict that protection of hotspots outperforms other area closures in safeguarding threatened pelagic predators from ecological extinction. We conclude that the seemingly monotonous landscape of the open ocean shows rich structure in species diversity and that these features should be used to focus future conservation efforts.
The oceanic pelagic ecosystem is by far the largest on Earth, covering >70% of the planet by area and an even larger percentage by volume. Human domination of this ecosystem is expanding rapidly as fishing fleets reach even the remotest areas (1). Serious conservation concerns arise as many large predators such as tuna and billfishes (2), sharks (3), and turtles (4) are driven to dangerously low levels, either as target catch or bycatch. Apart from the risk of species extinction (5), there are some wider ecosystem concerns. Consumers can play an important role in maintaining aquatic diversity and ecosystem functioning (6, 7). These effects are particularly pronounced if strong interactors, or keystone species, are affected (8, 9). For example, the elimination of some large predators and herbivores from inshore areas has triggered top-down effects, which contributed to the loss of ecosystem services and collapse of some coastal ecosystems (10). We currently do not have sufficient data to judge whether this applies to oceanic ecosystems (11), but if similar changes occur, they are bound to be massive in scale and probably difficult to reverse. Because of these concerns, marine scientists have been calling for large-scale protected areas in the open ocean (12, 13). In terrestrial ecosystems, the conservation of biodiversity hotspots has been identified as an effective way of protecting many species at once (14, 15). This concept was recently extended to coral reefs, which represent benthic biodiversity hotspots (16). Here we identify pelagic biodiversity hotspots in the open ocean using the best available data. Then we use simple closed-area models to explore whether closure of a hotspot to fishing represents a viable conservation option.
Methods
Data Sources. We compiled scientific-observer data from U.S. and Australian longline fisheries collected between 1991 and 2000. These consist of counts of all large tuna, billfishes, and other bony fish, sharks, sea turtles, seabirds, and marine mammals that are caught by pelagic longlines and recorded by independent, scientifically trained observers (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). Pelagic longlines are the most widespread fishing gear used in the open ocean. They are particularly useful for assessing pelagic diversity, because they catch a wide range of species in a similar way, over vast spatial scales, and are closely monitored by scientific observers. Their disadvantage is that they sample only larger species that are vulnerable to baited hooks. However, they do capture most known species that are of current conservation concern. Scientific observers record the number and status of each species caught as well as detailed information on gear and operational characteristics. Similar programs are maintained by the U.S. National Marine Fishery Service in the northwest Atlantic (Atlantic observer data since 1991, n = 1,962 longline sets) and around Hawaii (Hawaiian observer data since 1994, n = 3,290 sets) and by the Australian Fisheries Management Authority (Australian observer data since 1991, n = 3,127 sets). Observers identified 93 (Atlantic), 71 (Hawaii), and 69 (Australia) species or species groups, respectively (Table 1). We present data from the swordfish fishery in the northwest Atlantic, swordfish and tuna fishery in Hawaii, and tuna fishery in Australia. All data underwent extensive checks for consistency and robustness. Before we combined data for different target fisheries, we plotted data for each separately to confirm that they were showing the same patterns. Longline sets targeting sharks were excluded because of different fishing techniques (mostly bottom longlines) as compared with the tuna and swordfish fishery (pelagic longlines). Unidentified animals as well as sets with obvious coding errors (e.g., on land) were not considered. We also excluded sets beyond the euphotic zone (i.e., deeper than 200 m), because we assumed that these would sample a different community of species. These procedures did not change the results but improved the clarity of the patterns. In the northwest Atlantic, we also used longline vessel logbook data (1992–1999) to check for consistent patterns. Logbook data have a better sample size and spatial coverage (n = 62,001 sets) but lower taxonomic resolution than observer data (38 species distinguished; Table 1).
Mapping. For mapping diversity, we binned data into 2° by 2° hexagonal cells, excluding cells with less than three observed longline sets. The number of recorded species was a nonlinear function of fishing effort, saturating between 105 and 106 hooks per cell (Fig. 4, which is published as supporting information on the PNAS web site). We used standard rarefaction techniques to account for differences in fishing effort among cells and estimated two common indices of species diversity: species richness (the number of species per 50 individuals) and species density (the number of species per 1,000 hooks) in each cell (17). The rarefaction model is based on the hypergeometric distribution, sampling without replacement from a parent distribution. It is widely used to compare the number of species in a collection of samples with uneven sample sizes (17). Species richness is expressed as the expected number of species from a standardized subsample of size n, which is computed as
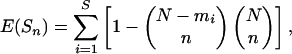 |
[1] |
where N is the total number of individuals in the sample, S is the total number of species in the sample, and mi is the number of individuals of species i in the sample (17). Species density is calculated as the expected number of species per 1,000 hooks. In this case, the number of individuals per 1,000 hooks determines n. We chose 50 individuals and 1,000 hooks as standardized subsample sizes because they correspond to the average number of individuals and hooks sampled by a single longlining set (see Table 1). These two measures of species diversity were highly correlated (0.92 > r2 > 0.55, P < 0.0001 in all cases), and the location of hotspots was the same; therefore we report species-richness patterns only. We performed extensive checks for consistency and robustness to make sure that observed patterns were not sensitive to changes in model parameters. (i) We varied the number of individuals in the standardized sample from 10 to 30, 50, 100, 200, and 500 individuals. (ii) To check for possible effects of fishing depth, we plotted data for longline sets above 50, 100, and 200 m in the Hawaiian fishery, which shows the greatest depth range and provides the best depth data. (iii) To check for possible effects of spatial scale, we binned data into 1° × 1°, 2° × 2°, and 3° × 3° cells, respectively. (iv) To test for seasonal changes in hotspots, we split the Atlantic logbook data, which was the only data set large enough to be divided, into four seasons. The latitudinal patterns and location of major hotspots were confirmed in all of these analyses. Relationships between latitude and diversity and catch rates and diversity were tested by using linear and second-order polynomial regression. We checked results by fitting maximum-likelihood estimates of spatial regression models to account for possible spatial dependence among cells by using a conditional autoregressive model (18). Spatial correlation C was alternatively assumed to affect only neighboring cells or to decline with distance d such that C = e(–d/D), where D was assumed to be 500 km (19).
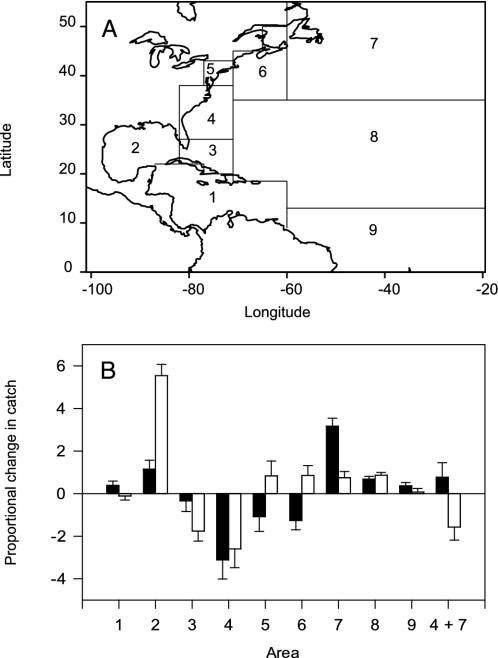
Closed-Area Model. In the northwest Atlantic, where we have the best data, we used an established closed-area model (3) to check whether the closure of a diversity hotspot is a superior conservation option compared with other area closures. The model was based entirely on empirical data (distribution of fishing effort from logbook, catch rates per species from observer data, pooled from 1992 to 1999). Theoretical annual closures of each area were considered under two contrasting scenarios: (i) after the closure fishing effort is displaced and changes such that the same target catch is maintained (“constant-quota scenario”), and (ii) fishing effort is displaced but remains constant (“constant-effort scenario”). Under both assumptions, effort is allocated from the closed area x to the remaining open areas i ≠ x based on the proportion of total swordfish catch Pi,x caught in area i, if area x is closed, i.e.,
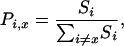 |
[2] |
where Si is the swordfish catch in area i. This means that fishers were assumed to reallocate effort from the closed area primarily to those open areas that support large swordfish catches. Further model details are described elsewhere (3). The northwest Atlantic was divided into nine management areas (Fig. 1A), of which one (area 7) has been closed since July 2001 to reduce bycatch of endangered sea turtles. For our model we expanded area 4 to cover the entire hotspot off the U.S. southeast coast (Fig. 1 A). Then we examined the effects of closing this and each of the remaining management areas on projected change in catches of 10 finfish, 2 turtle, and 10 shark species for which we had sufficient data. We used a minimum sample size of 25 individuals including finfish (Atlantic sailfish, white marlin, blue marlin, swordfish, Atlantic bluefin tuna, bigeye tuna, albacore tuna, common dolphinfish, wahoo, and oilfish), sea turtle (loggerhead and leatherback), and shark (great hammerhead, scalloped hammerhead, bignose, dusky, night, oceanic whitetip, silky, blacktip, bigeye thresher, and tiger) species (see Table 1 for scientific names). We chose these species because they are of particular conservation concern because of targeted overfishing, high bycatch rates, or declining population trends (3, 20, 21). To compare the overall effects of different closure scenarios across all species, we summed the proportional changes in catch for all species of conservation concern under the constant-quota and constant-effort scenarios, respectively.
Fig. 1.
Closed-area model. (A) Management areas in the northwest Atlantic were modified after the U.S. National Marine Fisheries Service (NMFS) classification for pelagic longline fisheries. The northward and southward boundaries of area 4 were expanded by 3° to cover the entire diversity hotspot shown in Fig. 2 A and B. Our areas 8 and 9 comprise two NMFS areas each. (B) Effects of closed areas on the summed proportional changes in catch for species of conservation concern in the northwest Atlantic. Results for the constant-quota (black bars) and constant-effort (white bars) scenarios are presented. Negative values refer to reductions in catch relative to the 1990s. Error bars are 95% bootstrap confidence intervals, accounting for the uncertainty in the observer estimates of species composition.
Results and Discussion
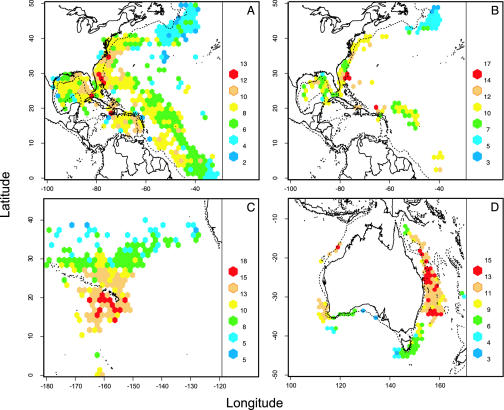
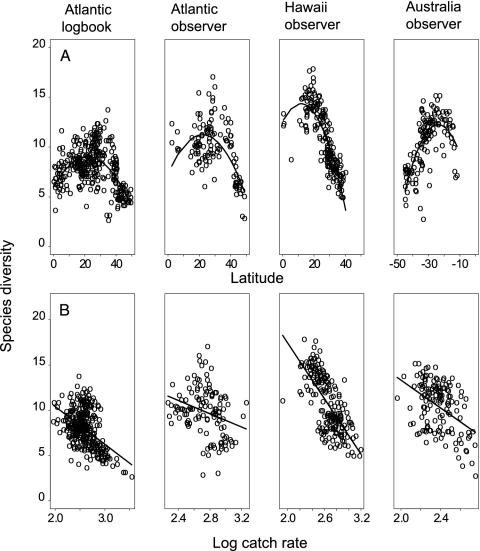
In all four data sets, species diversity peaked consistently at intermediate latitudes and close to prominent topographic features such as islands (Hawaii and Great Barrier Reef in Australia), shelf breaks (northwest Atlantic and northeast Australia), or seamounts (Hawaii and southeast Australia) (Fig. 2). High latitudes were characterized by low diversity, and tropical regions showed intermediate diversity. These patterns resulted in consistent unimodal relationships between latitude and diversity (Fig. 3A). Within a latitudinal band, diversity appeared to be highest along the shore and declined toward offshore regions. Around islands such as the Hawaiian Islands and the Antilles, diversity was often highest on the leeward side, relative to the main wind and current direction. Four major hotspots were located off the east coast of Florida (Fig. 2 A and B), south of Hawaii (Fig. 2C), and off the Great Barrier Reef and Lord Howe Island (Fig. 2D). Other regions of high diversity were found off the Carolinas and Greater Antilles (Fig. 2 A and B), and off the Australian northwest coast, which is close to a known tuna spawning area (22). Patterns found in the observer and logbook data in the northwest Atlantic were broadly similar for the major hotspot off the U.S. southeast coast but less consistent for areas with limited observer data such as the Antilles. Most hotspot regions show high diversity but sustain relatively low longline catch rates (Fig. 3B), which may suggest that fish productivity in hotspot regions is not high. In contrast, high catch rates are found in areas of low diversity, which are often located at high latitudes.
Fig. 2.
Predator diversity in the ocean, predicted from the northwest Atlantic longline logbook (A), observer data (B), Hawaiian observer data (C), and Australian observer data (D). Color codes indicate levels of species diversity calculated by rarefaction and expressed as the expected number of species per 50 individuals. Red cells indicate areas of maximum diversity, or hotspots. The dotted lines represent 1,000-m isobaths, identifying the outer margins of continental slopes.
Fig. 3.
Relationships between predator diversity (species per 50 individuals) and latitude (A) and log catch rate (number of individuals per 10,000 hooks) (B). Lines indicate second-order polynomial (latitude) and linear regression (log catch rate) fits. Best fits were tested by using spatial regression models. In all cases the quadratic term shown in A and the linear term shown in B were highly significant (P < 0.001) with the exception of the northwest Atlantic observer data shown in B (P < 0.07).
Biodiversity hotspots have been identified as conservation priorities in terrestrial ecosystems. In the marine realm, this could be different, because animals move extensively between areas (23), as does fishing effort (24). Management measures that displace fishing effort from a closed area may even cause harm, if that area was a prime fishing ground, and effort needs to be increased substantially to catch the same quota elsewhere. Results from a closed-area model of the northwest Atlantic (Fig. 1 A) predict that hotspot closure (area 4) is not only the best conservation option but is also the only option that would result in significant conservation benefits under both the constant-quota and constant-effort scenarios (Fig. 1B). These benefits result from a combination of two mechanisms: (i) many co-occurring species (sharks and finfish in particular) experience moderate to large reductions in catch when area 4 is closed, and (ii) catch rates for the target species are relatively low in that area, thus little effort needs to be reallocated elsewhere. These results suggests that, at least in the northwest Atlantic, pelagic diversity hotspots may be important conservation options, as they are on land (14, 15). Closing other areas to longlining had variable effects, which ranged from a weak decrease (area 3) to marked increases (areas 2 and 7) in catch. For example, the currently enforced closure-area 7 was predicted to reduce turtle bycatch (3) but may cause significant harm otherwise, particularly under the constant-quota scenario (Fig. 1B). The reason for this is that this area sustains the highest target catch rates, which generates large effort reallocation after a closure. Closing the hotspot (area 4) while keeping area 7 closed is still a good conservation option under the constant-effort scenario but performs poorly under constant quotas (Fig. 1B). We point out that these particular predictions, particularly for the constant-quota scenario, should be treated with caution because 40% of swordfish catches are currently taken in areas 4 and 7. However, these results suggest that it may be difficult to protect biodiversity through multiple large-scale closures without controlling fishing effort at the same time.
Our analysis indicates that large oceanic predators occur in distinct diversity hotspots that are characterized by extraordinary diversity. We do not know at present whether hotspots may have been more abundant, larger, or located elsewhere in a pristine ocean. Using data from the 1990s, we describe those hotspots, which remain after decades of human alteration of ocean ecosystems (25). On an ocean-basin scale, diversity seemed to peak consistently between 20° and 30° latitude (Fig. 3A). Seasurface temperatures in these regions range from ≈20°Cto26°C, which may be favorable for both temperate and tropical species, increasing regional diversity. Remarkably, very similar latitudinal patterns of species diversity are seen in five major zooplankton taxa: fish larval, ostracod, decapod, and euphausiid species richness peaked at 20° latitude along a northeast Atlantic transect (26), and planktic foraminiferal richness consistently peaked at 20–30° latitude in all oceans (27). Within these latitudinal bands, clear foraminiferal hotspots were found around Hawaii and off Florida (27). These patterns suggest that hotspot regions may overlap for disparate trophic groups. On a local scale, diversity hotspots consistently seem to be associated with prominent topographic features such as reef islands, shelf breaks, or seamounts. Oceanographically, these features are characterized by increased turbulence, mixing, and mesoscale eddies, which can enhance local production by transporting nutrients into the euphotic zone (28, 29). In addition, they also tend to concentrate food supply and have been shown to provide key feeding areas for pelagic species (30–32). Diversity hotspots in the northwest Atlantic as well as Hawaii coincide conspicuously with peaks of eddy kinetic energy (29, 33), which may further underline the importance of these regional oceanographic features. Our diversity analysis implies that in food-stressed habitats such as the open ocean, these features may be critical to a large number of species. In addition, three of the four hotspots were found within or directly adjacent to recently identified coral reef hotspots, located around the Great Barrier Reef, Lord Howe Island, and the Hawaiian Islands (16). We hypothesize that the rich habitat structure and dynamic oceanographic conditions associated with coral reefs (28) may favor adjacent pelagic hotspots, which may mean that efforts to conserve existing coral hotspots, for example in the Great Barrier Reef Marine Park, should consider expanding protection into adjacent pelagic waters.
We conclude that the protection of pelagic diversity hotspots may represent an important conservation option, because many species benefit concurrently. In addition to conservation benefits, the maintenance of high diversity may also be critical for the sustainability of fishing (34). Our results further emphasize that the siting of marine protected areas is of critical importance, because some areas may cause indirect harm through uncontrolled displacement of fishing effort. We suggest that the identification and conservation of hotspots, in concert with reductions in fishing effort, may represent an important tool for protecting threatened pelagic predators from further declines and ecological extinction.
Supplementary Material
Acknowledgments
We thank S. Harley and P. Doherty for critical advice and help with the modeling; J. Baum, G. Rilov, N. Shackell, and P. Ward for comments; W. Blanchard for statistical advice; D. Swan for technical help; and the U.S. National Marine Fisheries Service and Australian Fisheries Management Authority for data. This research is part of a larger project on pelagic longlining initiated and supported by Pew Charitable Trusts. Additional support was received from the German Research Council, the Killam Foundation, the Natural Science and Engineering Research Council of Canada, and the Future of Marine Animal Populations program, funded by the Sloan Foundation.
References
- 1.Botsford, L. W., Castilla, J. C. & Peterson, C. H. (1997) Science 277, 509–515. [Google Scholar]
- 2.Safina, C. (1998) Song for the Blue Ocean (Holt, New York).
- 3.Baum, J. K., Myers, R. A., Kehler, D., Worm, B., Harley, S. J. & Doherty, P. A. (2003) Science 299, 389–392. [DOI] [PubMed] [Google Scholar]
- 4.Spotila, J. R., Reina, R. D., Steyermark, A. C., Plotkin, P. T. & Paladino, F. V. (2000) Nature 405, 529–530. [DOI] [PubMed] [Google Scholar]
- 5.Casey, J. M. & Myers, R. A. (1998) Science 281, 690–692. [DOI] [PubMed] [Google Scholar]
- 6.Paine, R. T. (2002) Science 296, 736–739. [DOI] [PubMed] [Google Scholar]
- 7.Worm, B., Lotze, H. K., Hillebrand, H. & Sommer, U. (2002) Nature 417, 848–851. [DOI] [PubMed] [Google Scholar]
- 8.Paine, R. T. (1966) Am. Nat. 100, 65–76. [Google Scholar]
- 9.Power, M., Tilman, D., Estes, J., Menge, B., Bond, W., Mills, L., Daily, G., Castilla, J., Lubchenco, J. & Paine, R. (1996) Bioscience 46, 609–620. [Google Scholar]
- 10.Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., et al. (2001) Science 293, 629–638. [DOI] [PubMed] [Google Scholar]
- 11.Kitchell, J. F., Boggs, C., He, X. & Walters, C. (1999) in Ecosystem Approaches for Fisheries Management (Alaska Sea Grant College Program, Fairbanks, AK), pp. 665–685.
- 12.Mills, C. E. & Carlton, J. T. (1998) Conserv. Biol. 12, 244–247. [Google Scholar]
- 13.Hyrenbach, K. D., Forney, K. A. & Dayton, P. K. (2000) Aquatic Conserv. Mar. Freshw. Ecosyst. 10, 437–458. [Google Scholar]
- 14.Pimm, S. L. & Lawton, J. H. (1998) Science 279, 2068–2069. [Google Scholar]
- 15.Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. (2000) Nature 403, 853–858. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, C. M., McClean, C. J., Veron, J. E. N., Hawkins, J. P., Allen, G. R., McAllister, D. E., Mittermeier, C. G., Schueler, F. W., Spalding, M., Wells, F., et al. (2002) Science 295, 1280–1284. [DOI] [PubMed] [Google Scholar]
- 17.Gotelli, N. J. & Graves, G. R. (1996) Null Models in Ecology (Smithsonian Institution Press, Washington, DC).
- 18.Cressie, N. A. C. (1993) Statistics for Spatial Data (Wiley, New York).
- 19.Myers, R. A., Mertz, G. & Bridson, J. M. (1997) Can. J. Fish. Aquat. Sci. 54, 1400–1407. [Google Scholar]
- 20.U.S. National Marine Fisheries Service (2002) Stock Assessment and Fishery Evaluation for Atlantic Highly Migratory Species 2002 (U.S. National Marine Fisheries Service, Highly Migratory Species Management Division, Silver Spring, MD).
- 21.Hilton-Taylor, C. (2000) 2000 IUCN Red List of Threatened Species (World Conservation Union, Gland, Switzerland).
- 22.Caton, A. E. (1991) Inter-Am. Trop. Tuna Comm. Spec. Rep. 7, 181–350. [Google Scholar]
- 23.Block, B. A., Dewar, H., Blackwell, S. B., Williams, T. D., Prince, E. D., Farwell, C. J., Boustany, A., Teo, S. L. H., Seitz, A., Walli, A. & Fudge, D. (2001) Science 293, 1310–1314. [DOI] [PubMed] [Google Scholar]
- 24.Murray, K. T., Read, A. J. & Solow, A. R. (2000) J. Cetacean Res. Manag. 2, 135–141. [Google Scholar]
- 25.Myers, R. A. & Worm, B. (2003) Nature 423, 280–283. [DOI] [PubMed] [Google Scholar]
- 26.Angel, M. V. (1997) in Marine Biodiversity, eds. Ormond, R. F. G., Gage, J. D. & Angel, M. V. (Cambridge Univ. Press, Cambridge, U.K.), pp. 35–69.
- 27.Rutherford, S., D'Hondt, S. & Prell, W. (1999) Nature 400, 749–753. [Google Scholar]
- 28.Wolanski, E. & Hamner, W. M. (1988) Science 241, 177–181. [DOI] [PubMed] [Google Scholar]
- 29.Oschlies, A. & Garçon, V. (1998) Nature 394, 266–269. [Google Scholar]
- 30.Haney, J. C. (1986) Mar. Ecol. Prog. Ser. 28, 279–285. [Google Scholar]
- 31.Fonteneau, A. (1998) Int. Comm. Conserv. Atl. Tunas Tuna Symp. L (1), 275–317. [Google Scholar]
- 32.Weimerskirch, H., Bonadonna, F., Bailleul, F., Mabille, G., Dell'Omo, G. & Lipp, H.-P. (2002) Science 295, 1259. [DOI] [PubMed] [Google Scholar]
- 33.Flament, P., Kennan, S., Lumpkin, R., Sawyer, M. & Stroup, E. D. (1996) The Ocean Atlas of Hawaii (School of Ocean and Earth Science and Technology, Univ. of Hawaii, Honolulu), http://satftp.soest.hawaii.edu/atlas.
- 34.Hilborn, R., Quinn, T. P., Schindler, D. E. & Rogers, D. E. (2003) Proc. Natl. Acad. Sci. USA 100, 6564–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.