Abstract
Over the period 1953–1979, a battery factory on the Hudson River in New York released ≈53 tons of cadmium (Cd) and nickel hydride wastes into Foundry Cove. The most common aquatic benthic species, the oligochaete Limnodrilus hoffmeisteri, rapidly evolved resistance to Cd. The capacity for detoxification and internal storage of Cd resulted in a strong potential for trophic transfer of Cd through the aquatic food web. As a result of United States Superfund legislation, a major remediation effort in 1994–1995 removed the majority of the Cd, thereby removing the selective force for resistance. The cleanup of this cove resulted in the maintenance of resistant forms but then there ensued a rapid loss of resistance in ≈9–18 generations, showing the potential for ecological restoration to rapidly reduce the potential for trophic transfer of Cd through the ecosystem. This study demonstrates a genetic approach to the study of ecological restoration and connects a genetic indicator of restoration to transfer of toxic metals through ecosystems.
So many ecosystems have been polluted and altered by human activities that environmental restoration is now a major objective facing society. In the United States, the Superfund Act of 1980 was passed specifically to provide guidance for restorations and cleanups. It is of crucial importance to provide scientific criteria for restoration that objectively assess the degree of ecological recovery. Human activities strongly affect genetic variation in natural populations (1), which makes evolutionary change a natural focus of restoration investigations. Toxic substances such as pesticides and metals generate among the most powerful of these selective forces, on both targeted pests and on nontarget species, whose physiological and ecological traits and position in food chains make them vulnerable to exposure. Large quantities of metals have been introduced into aquatic ecosystems and, as a result, most waterways have dissolved and particulate concentrations of metals that exceed accepted health or other environmental guidelines (2, 3). Owing to their strong toxic effects, natural selection favors genotypes that increase the resistance of members in the local population. There is now extensive evidence that releases of metals have resulted in the development of metal resistance in many terrestrial and aquatic populations (4, 5). Aquatic and marine environments exposed to metal pollution are known to harbor natural populations that are unusually resistant to metals (6–11).
Foundry Cove, a tidal freshwater estuarine cove located near Cold Spring, New York, was one of the most metal-polluted areas in the world. From 1953 to 1979 a battery factory released an estimated 53 tons of cadmium (Cd) and nickel hydride wastes into the cove, resulting in sediment concentrations of >10,000 ppm dry weight of Cd throughout much of the cove and concentrations as high as 250,000 ppm Cd near the pipe outfall (12). We investigated the resistance to Cd of the most common invertebrate, the tubificid oligochaete Limnodrilus hoffmeisteri. This abundant deposit feeder is likely the most important organism involved in sediment turnover and the most abundant benthic faunal food source for higher trophic levels (13, 14). We found very high resistance in this species. Nearly all of the variation in resistance could be explained by genetic variation, probably segregating at one locus (6, 15). Resistant worms synthesized large amounts of a metal-binding protein of low molecular weight, but nonresistant worms produced <10% of this amount, even when exposed to the same Cd dose (13, 16). Comparable populations from a nearby unpolluted cove or even from sites within Foundry Cove inhabiting sediments with lower levels of Cd were not resistant, and even grandchildren of Foundry Cove worms raised in clean sediment through a third generation were still found to be nearly as resistant as worms collected directly from the cove (6). Laboratory selection experiments and measurements of parent–offspring resemblance of Cd resistance both demonstrated high narrow-sense heritabilities, estimated to be ≈0.7–1. The measured mortality rates, combined with measured heritabilities, suggested that the evolution of resistance in Foundry Cove was very rapid, perhaps only in one to four generations (6).
In 1989, the United States Environmental Protection Agency published a Record of Decision, consistent with the United States 1980 Superfund Act, to remove most of the Cd contamination from Foundry Cove sediments. When the cleanup commenced in 1994, sediments still had Cd concentrations of 2,000 ppm and worms from the most polluted parts of Foundry Cove were still more resistant to Cd than worms collected from nearby South Cove (17). We measured the resistance of L. hoffmeisteri over a period of 9 yr, starting 1 yr before the cleanup, by exposing worms from Foundry Cove as well as worms from unpolluted South Cove, which is 1 km to the south, to a test solution of dissolved Cd. Because we demonstrated that the difference in mortality rate was genetically determined and had differed between the formerly polluted Foundry Cove and the unpolluted South Cove (6, 15), we elected to use this same bioassay after the cleanup, which immediately brought the sediment concentrations of Cd before the cleanup to concentrations of <10 ppm. This concentration was far below those that we ever observed increased resistance to Cd before the cleanup (6, 14). Our principal objectives were to determine whether (i) the Foundry Cove populations lost resistance, and, if so, (ii) how rapidly the genetically based recovery happened.
Methods
Previous work (6) demonstrated that mortality rate in response to metal exposure was a highly heritable trait, and we therefore used the same measure to follow loss of resistance to Cd after the cleanup. Sediment was collected from Foundry Cove and South Cove in late July or August of each year that had been studied and described (6, 13, 15). Owing to time constraints, we sampled minimally from one site in each cove. In 1996, after the cleanup, we sampled at a formerly high-Cd site (FCa)of ≈10,000 ppm and another with lower sediment concentrations of ≈500 ppm (FCb) before the cleanup. In all years but 2002, we sampled from one site in South Cove (SCa), but in 2002 we sampled from one other site ≈100 m to the north (SCb). We washed the sediment through a 250-μm mesh and picked worms from each locality by using a pipette. Within 2–3 days of field collection, we exposed ≈100 worms from each site to 8.9 μM Cd, dissolved in reconstituted soft water (18). This concentration is sufficient to eventually kill all of the worms in the experimental treatment, irrespective of site of origin. Each worm was kept in a separate well (4 ml of Cd solution) at room temperature. Every hour we checked for mortality and followed the experiment until half of the worms had died. We therefore measured the rate of mortality. Mortality curves from different experimental populations were compared statistically by means of a Wilcoxon ranked-pairs nonparametric test (19), which compared death in any two experimental worm populations at different successive hours, until half of the population had expired.
Cd analyses were performed by using a graphite furnace atomic absorption spectrophotometer following previously published methods for sediment (14) and L. hoffmeisteri body concentrations (18).
Results
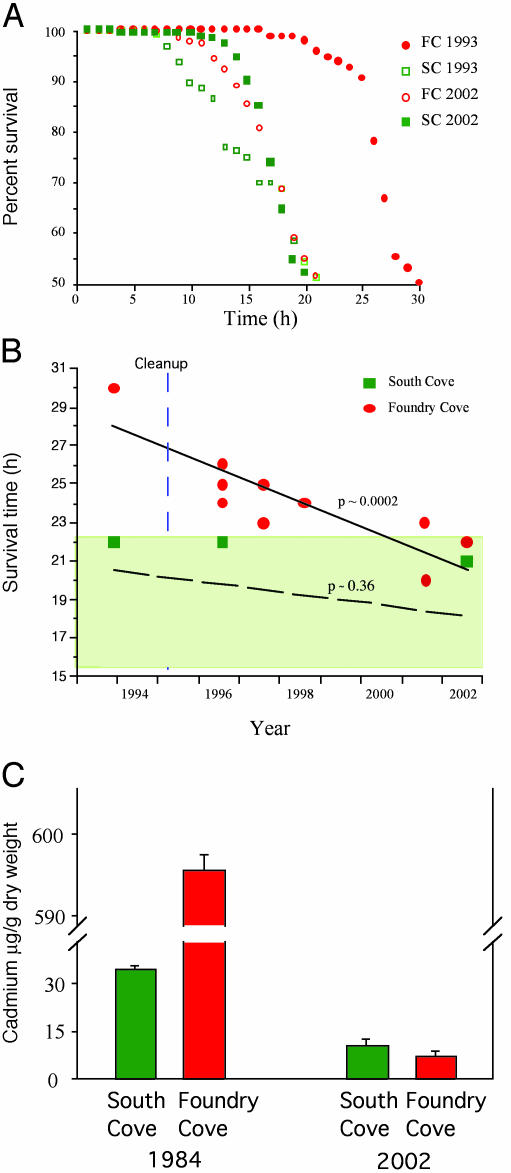
Fig. 1 A and B shows the results of the mortality experiments. Before the cleanup, Foundry Cove worms were far more resistant to Cd than worms from the unpolluted South Cove. In the first year after the cleanup, Foundry Cove worms were still more resistant than South Cove worms, even though Foundry Cove sediment Cd concentrations were now approximately equal to those of the relatively unpolluted South Cove (20), and far less than the concentrations required to cause the evolution of resistance. By 2001, we found that sediment Cd concentrations at Foundry Cove and South Cove were both <6 ppm (14). Thus the significant difference in resistance between Foundry Cove and South Cove worms during the period 1996–2001 could not be explained by a nongenetic effect of induction of metal-binding proteins in Foundry Cove animals because the metals were nearly gone yet the difference in resistance persisted. By the summer of 2002, however, resistance to Cd of Foundry Cove worms could no longer be distinguished from that of worms living in the unpolluted cove (Fig. 1 A). From 1993 to 2002, resistance declined steadily, whereas there was no significant temporal trend in the resistance level of South Cove worms to the same exposure concentrations of Cd (Fig. 1B and Table 1). Relative to original surveys in the 1980s, when Cd body burdens of Foundry Cove worms were very high, we found no difference between the body burdens of worms collected in 2002 from Foundry Cove and South Cove (Fig. 1C).
Fig. 1.
Loss of resistance of the oligochaete L. hoffmeisteri after a Cd cleanup. (A) Comparison of mortality curves for Foundry Cove and South Cove L. hoffmeisteri in 1993, just before the cleanup, and in August 2002. (B) Change in time to 50% mortality after Cd exposure for Foundry Cove and for unpolluted South Cove, before and after the cleanup. Lines show model I regression best fits of trends in change of resistance over time. Probabilities for ANOVA of regression are also shown. (C) Cd concentrations of Foundry Cove L. hoffmeisteri in 1984 and in 2002, 8 yr after the cleanup, in Foundry Cove and South Cove.
Table 1. Statistical comparisons of survival.
| Date | Comparisons between Foundry Cove and South Cove |
|---|---|
| 1993 | FCa > SCa |
| 1996 | FCa > FCb > SCa |
| 1997 | FCa > SCa |
| 1998 | FCa > SCa |
| 2001 | FCa > FCb > SCa |
| 2002 | FCa = FCb = SCa = SCb |
Comparisons of mean survival function between locations in Foundry Cove (FC) and South Cove (SC) after the restoration by using generalized Wilcoxon tests. >, significant difference (P < 0.01); =, no significant difference (P > 0.05).
Discussion
The results demonstrate a rapid loss of genetically based resistance after the Superfund cleanup. Our data on abundance change and other life history studies suggest that L. hoffmeisteri reproduces one to two times a year and that sexual maturity can be reached in 1 yr (21–23). Thus, we can assign an upper conservative limit of 9–18 generations for the complete loss of resistance, which is a little more than three to four times the estimated time that it took to evolve resistance in the first place (6). In the year 2002, the population density of L. hoffmeisteri in Foundry Cove was still less than that of South Cove (14). The difference, however, may relate more to change in sedimentary properties than to Cd concentrations, because the sediment surface in Foundry Cove can be shown to be significantly more compacted than the otherwise comparable South Cove (14).
Reversion to lowered tolerance of Cd by Foundry Cove worms could be explained by either a trade-off between Cd adaptation and population growth rates or by dispersal of large numbers of nonresistant genotypes into the cove from the Hudson River, after the cleanup. The major source of recruits would be from the single major tidal exchange, through a narrow channel to the main part of the Hudson River. Oligochaetes lack a planktonic larval stage but may emerge from the sediment and can be transported by means of water currents, as has been found for other aquatic invertebrates (24). Nevertheless, after two deployments of plankton nets at this entrance in December 1999 and August 2002, each for 1 h, we found no evidence of such transport. Although these dates represent winter and summer sampling, it is still possible, yet unlikely, that worms disperse at other times of year.
It is more probable that the loss of resistance in Foundry Cove worms is due to a trade-off between adaptation to Cd and some life history trait. Resistant genotypes in sediments with high Cd could grow and reproduce, but they had very slow somatic growth rates, and metal-exposed individuals may have diverted resources from growth to make high concentrations of a metal-binding metallothionein-like protein, which we have found to be mainly a genetically determined trait in Foundry Cove worms (16). A laboratory study of increase of Cd resistance in Drosophila melanogaster demonstrated trade-off between increased survival and life history factors such as mortality rate, which would lead to a rapid loss of Cd-adapted genotypes when selection was relaxed (25). In metal-adapted grasses, recovery after land restoration might occur because metal-resistant genotypes are poor competitors with con-specifics that are metal-intolerant (26).
The steady loss of resistance in Foundry Cove L. hoffmeisteri after the cleanup was clearly due to change in a genetically based trait in the population. Our previous quantitative genetic work demonstrates that nearly all of the difference in ability to resist Cd poisoning and to rapidly produce large amounts of a metallothionein-like protein was due to genetic differences between Foundry Cove and South Cove worms and not just induction by Cd (6, 16). Sediment concentrations of as much as 500 ppm failed to select for resistance (6). Just after the cleanup, sediment concentrations were extremely low, averaging <10 ppm (20), and yet Foundry Cove worms were still more resistant than South Cove worms (Fig. 1). Thus, there was a lag time before resistance was lost, further demonstrating that the loss of resistance was a genetic process.
This study shows that recovery from severe pollution can be very rapid and demonstrates the ability of a natural population to recover and lose resistance after a major and disruptive cleanup. The disappearance of resistant genotypes was accompanied by other signs of recovery. The sediment was substantially lower in Cd concentration, and Cd body burden of L. hoffmeisteri is now very low and indistinguishable from those of South Cove worms (Fig. 1C). The process of reversal of resistance, therefore, has had a potential large-scale ecological effect because resistant genotypes transfer Cd more efficiently through the food web (17) and affect both the Cd concentrations and prey-location ability of predators (27).
The evolution of resistance is a well known theme in a wide range of populations exposed to toxic substances ranging from pesticides to metals (5, 28, 29). Natural selection is powerful, and variation at single or very few loci can be selected very rapidly (15, 30). Our previous work demonstrates that the evolutionary response to metals was rapid and probably based on selection for increased expression of a metal-binding protein (6, 16). However, this work demonstrates that reversal can also be a matter of a few generations and that such reversal is a good measure of restoration in the form of removal of potential for trophic transfer of metals. It is therefore important to assess the extent of the potential for natural selection and also the potential for genetic recovery when an area is restored. In the case of Foundry Cove, nearly 100 million dollars was spent on the cleanup. Our study shows that environmental restorations may cause rapid genetic recoveries. Our results are consistent with a rapid recovery in resistance previously found when the use of the insecticide DDT was relaxed against the mosquito Aedes aegypti (28).
Acknowledgments
This paper is dedicated to the memory of James Rod, whose dedication led to the cleanup and restoration of Foundry Cove. We are grateful to the staff of the Constitution Marsh Audubon Sanctuary for their support and facilities. We thank the Hudson River Foundation for support of this research.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Palumbi, S. R. (2001) The Evolution Explosion: How Humans Cause Rapid Evolutionary Change (W.W. Norton, New York).
- 2.Segar, D. A. & Davis, P. G. (1984) in NOAA Technical Report Memorandum (Natl. Oceanic and Atmos. Admin., Rockville, MD), Vol. NOS OMA 11, pp. 1–120. [Google Scholar]
- 3.U.S. Environmental Protection Agency (2000) National Water Quality Inventory, 2000 Report, 305b (U.S. Environ. Protection Agency, Washington, DC).
- 4.Macnair, M. R. (1987) Trends Ecol. Evol. 2, 354–359. [DOI] [PubMed] [Google Scholar]
- 5.Klerks, P. L. & Weis, J. S. (1987) Environ. Pollut. 45, 173–205. [DOI] [PubMed] [Google Scholar]
- 6.Klerks, P. L. & Levinton, J. S. (1989) Biol. Bull. (Woods Hole, Mass.) 176, 135–141. [Google Scholar]
- 7.Weis, J. S., Weis, P., Heber, M. & Vaidya, S. (1981) Mar. Biol. (Berlin) 65, 283–287. [Google Scholar]
- 8.Lavie, B. & Nevo, E. (1986) Mar. Pollut. Bull. 17, 21–23. [Google Scholar]
- 9.Nevo, E., Ben-Shlomo, R. & Lavie, B. (1984) Proc. Natl. Acad. Sci. USA 81, 1258–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan, G. W. & Hummerston, L. G. (1973) J. Mar. Biol. Assoc. U.K. 53, 839–857. [Google Scholar]
- 11.Brown, B. E. (1976) Water Resour. Bull. 10, 555–559. [Google Scholar]
- 12.Knutson, A. B., Klerks, P. L. & Levinton, J. S. (1987) Environ. Pollut. 45, 291–304. [DOI] [PubMed] [Google Scholar]
- 13.Klerks, P. L. & Levinton, J. S. (1989) in Ecotoxicology: Problems and Approaches, eds. Levin, S. A., Kelley, J. R. & Harvell, M. A. (Springer, Berlin), pp. 41–67.
- 14.Kelaher, B. H., Levinton, J. S., Oomen, J., Allen, B. J. & Wong, W. H. (2003) Estuaries, in press.
- 15.Martinez, D. E. & Levinton, J. S. (1996) Evolution (Lawrence, Kans.) 50, 1339–1343. [DOI] [PubMed] [Google Scholar]
- 16.Klerks, P. L. & Bartholomew, P. R. (1991) Aquat. Toxico. 19, 97–112. [Google Scholar]
- 17.Wallace, W. G., Lopez, G. R. & Levinton, J. S. (1998) Mar. Ecol. Prog. Ser. 172, 225–237. [Google Scholar]
- 18.American Society for Testing and Materials (1996) ASTM Book of Standards E729–96, 1–22. [Google Scholar]
- 19.Lee, E. T. (1992) Statistical Methods for Survival Data Analysis (Wiley, New York), 2nd Ed.
- 20.Advanced Geoservices (2001) 5 Year Review. Long-Term Monitoring Program: Marathon Remediation Site (Advanced Geoservices, Chadds Ford, PA), pp. 1–17.
- 21.Poddubnaya, T. L. (1979) in Aquatic Oligochaete Biology, eds. Brinkhurst, R. O. & Cook, D. G. (Plenum, New York), pp. 175–184.
- 22.Block, E. M., Moreno, G. & Goodnight, C. J. (1982) Int. J. Invertebr. Reprod. 4, 239–247. [Google Scholar]
- 23.Kennedy, C. R. (1966) Oikos 17, 158–168. [Google Scholar]
- 24.Palmer, M. A. (1988) Mar. Ecol. Prog. Ser. 48, 81–91. [Google Scholar]
- 25.Shirley, M. D. F. & Sibly, R. M. (1999) Evolution (Lawrence, Kans.) 53, 826–836. [DOI] [PubMed] [Google Scholar]
- 26.Macnair, M. R. (1981) in Genetic Consequences of Man Made Change, eds. Bishop, J. A. & Cook, L. M. (Academic, London), pp. 177–207.
- 27.Wallace, W. G., Brouwer, T. M. H., Brouwer, M. & Lopez, G. R. (2000) Environ. Toxicol. Chem. 19, 962–971. [Google Scholar]
- 28.Wood, R. J. & Bishop, J. A. (1981) in Genetic Consequences of Man Made Change, eds. Bishop, J. A. & Cook, L. M. (Academic, London), pp. 97–127.
- 29.Daborn, P. J., Yen, J. L., Bogwitz, M. R., Le Goff, G., Feil, E., Jeffers, S., Tijet, N., Perry, T., Heckel, D., Batterham, P., et al. (2002) Science 297, 2253–2256. [DOI] [PubMed] [Google Scholar]
- 30.Ranson, H., Claudianos, C., Ortelli, F., Abgrall, C., Hemingway, J., Sharakhova, M. V., Unger, M. F., Collins, F. H. & Feyereisen, R. (2002) Science 298, 179–181. [DOI] [PubMed] [Google Scholar]