Abstract
Models predict that global warming may increase aridity in water-limited ecosystems by accelerating evapotranspiration. We show that interactions between warming and the dominant biota in a grassland ecosystem produced the reverse effect. In a 2-year field experiment, simulated warming increased spring soil moisture by 5–10% under both ambient and elevated CO2. Warming also accelerated the decline of canopy greenness (normalized difference vegetation index) each spring by 11–17% by inducing earlier plant senescence. Lower transpirational water losses resulting from this earlier senescence provide a mechanism for the unexpected rise in soil moisture. Our findings illustrate the potential for organism–environment interactions to modify the direction as well as the magnitude of global change effects on ecosystem functioning.
Global warming is expected to increase evapotranspiration, causing soil moisture declines that may offset modest increases in continental precipitation and lead to greater aridity in water-limited systems around the world (1–6). The models that predict this drying do not, however, incorporate direct biotic responses to warming. Several kinds of vegetation responses to warming, including changes in phenology, leaf area index, and rooting depth, could influence evapotranspiration rates and soil moisture availability (7–9).
Experimental observations of the effects of warming on ecosystem water balance, however, are scarce. In perennial-dominated arctic and alpine systems with short summer growing seasons, experimental warming has led to soil drying (10, 11). However, these effects appear to be mediated in part by warming-induced changes in the rate and timing of snowmelt as well as by increased evapotranspiration during the relatively brief time when plants are exposed.
For the snow-free majority of ecosystems in which vegetation interacts directly with the atmosphere for more of the year, biotic mechanisms could play important roles in mediating water balance. In particular, warming effects on plant production or phenology could influence soil moisture losses by changing the rate or annual duration of plant transpiration (12). We followed the responses of soil moisture availability and plant canopy phenology in a temperate grassland to 2 years of simulated warming under both ambient and elevated CO2. We present experimental evidence of a mechanism controlled by temperature responses of grassland vegetation, through which warming increases moisture availability at a critical period in a seasonally dry ecosystem.
Methods
Our study site was an annual-dominated California grassland at the Jasper Ridge Biological Preserve, Stanford, CA (37°24′N 122°13′W). In this region's Mediterranean-type climate, annual plants germinate with the onset of fall/winter rains. Plants set seed and senesce as water limitation becomes severe with cessation of rain in March–May. In 1997, we established 32 circular plots2min diameter and surrounded each with a solid below-ground partition to 50-cm depth. Since November 1998, we have exposed these plots to simulated warming (80 W m-2 of thermal radiation, resulting in a soil-surface warming of 0.8–1 C) and/or elevated CO2 (ambient + 300 ppm) in a 2 × 2 factorial design replicated eight times. We delivered warming with IR heat lamps suspended over plot centers. We elevated CO2 with a free-air ring of emitters around each plot delivering pure CO2 at the canopy level (13).
We used time domain reflectometry (14) to measure volumetric soil moisture approximately weekly from November 1998 to July 2000 and March to May 2001. Reported soil moisture values are for the top 15 cm of soil, which contains ≈85% of root biomass at our site (B. Shaw, personal communication). Previous studies have used a variety of approaches to detect acceleration of phenology as a result of warming in individual plant species (15–21). To capture the response of overall canopy phenology, we measured plot-scale normalized difference vegetation index (NDVI) with a portable spectroradiometer. Because NDVI is sensitive to the total chlorophyll content and fractional photosynthetic energy absorption of canopies (22–24), it serves as a reliable spatial and temporal indicator of canopy greenness, senescence, and phenology in grassland ecosystems (25–27). We calculated NDVI as (NIR – RED)/(NIR + RED), with NIR (near infrared) = average reflectance 720–740 nm and RED (visible red band) = average reflectance at 660–680 nm. Soil moisture and NDVI results were tested by using repeated-measures ANOVA with CO2 and heat as fixed factors (n = 32 plots). Post hoc tests of treatment effects on individual dates were performed by using ANOVA with CO2 and heat as fixed factors.
Results and Discussion
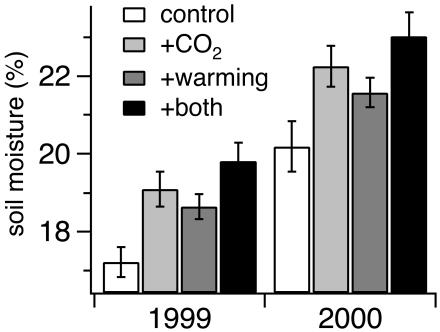
In both 1999 and 2000, warming and elevated CO2 treatments each increased soil moisture between mid-March and early July (Fig. 1). Across both years, mean stimulation of spring soil moisture by simulated warming (1.1 ± 0.95%) was comparable in magnitude to the mean stimulation of soil moisture by the elevated CO2 treatment (1.6 ± 1.0%). Combined warming and elevated CO2 enhanced mean spring soil moisture by 2.7 ± 1.1%, representing a 15% increase over baseline soil moisture availability during the transition from the rainy season to the summer drought.
Fig. 1.
Warming and elevated CO2 effects on spring soil moisture, 1999–2000. Values are mean soil moistures from January to July in each year. n = 20 and 15 dates for 1999 and 2000, respectively. Warming: P1999 = 0.016, P2000 = 0.065; elevated CO2: P1999 = 0.011, P2000 = 0.004.
Although elevated CO2 has been shown to increase soil moisture in other field experiments (28), stimulation of soil moisture by warming has not been reported. Several experiments have, however, detected warming-induced acceleration of flowering and/or senescence in plants the field (15–21). The yearly senescence of annual or seasonally dormant plants drastically reduces canopy conductance and lowers transpirational losses from the soil in other grasslands (29). Plant transpiration contributes >80% of total evapotranspiration in our study area during May–June (30), providing ample opportunity for warming effects on transpiration to affect overall water losses. Experimental warming at our site did not decrease total plant production in 1999 or 2000 (data not shown), ruling out the mechanism of reduced transpirational losses through overall production declines. We therefore hypothesized that warming caused by acceleration of canopy senescence increased moisture availability in our study system by reducing seasonal transpiration losses.
At our site, experimental warming accelerated senescence of the plant canopy in both years. In 1999, the warming treatment reduced the NDVI by 11–16% compared with unwarmed plots from late May to June (Fig. 1). Although its effect on the NDVI was not significant on any individual date in 2000, simulated warming reduced the NDVI by 12–17% from late May to mid-July. The course of the NDVI decline at the end of both growing seasons suggests that warming accelerates canopy senescence in this system. In both years, the NDVI decline slightly preceded the period of greater soil moisture under the warming treatment (Fig. 2). This timing is consistent with the mechanism we propose, in which the effects of reduced transpirational losses accumulate after warming pushes the canopy to earlier senescence.
Fig. 2.
Treatment effects on canopy NDVI (Upper) and soil moisture (Lower), 1999–2000. + and * denote significant effects of warming on individual dates at P = 0.10 and 0.05 levels, respectively.
Our findings illustrate the potential for organism–environment interactions to strongly modify global change effects on ecosystem function. We suggest that in at least some ecosystems, declines in plant transpiration mediated by changes in phenology can offset direct increases in evaporative water losses under future warming. Mediterranean-type ecosystems throughout the world experience similar growing season dynamics to our site and even share some common species as a result of exotic introductions (31, 32). It is reasonably likely that they will respond in qualitatively similar ways to warming. In many of the world's grasslands and savannas, subtle changes in resource availability at critical times can dramatically alter species composition (33). Extra moisture during the critical time of drought onset facilitates establishment of both late-season and woody plant species in California grasslands (33, 34). The biotic link between warming and water balance that we describe thus could influence community responses to climate change in this and other grasslands and savannas.
Acknowledgments
We thank the many volunteers and field assistants who contributed to this project. E.S.Z. received support from a U.S. Environmental Protection Agency STAR Fellowship, the Switzer Foundation, the A. W. Mellon Foundation, and a D. H. Smith Conservation Research Fellowship from The Nature Conservancy. B.D.T. received support from the A. W. Mellon Foundation. M.R.S. was supported by a Hollaender Postdoctoral Fellowship from the U.S. Department of Energy. The Jasper Ridge Global Change Experiment was supported by the National Science Foundation, the David and Lucile Packard Foundation, the Morgan Family Foundation, and the Jasper Ridge Biological Preserve.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: NDVI, normalized difference vegetation index.
References
- 1.Gregory, J. M., Mitchell, J. F. B. & Brady, A. J. (1997) J. Climate 10, 662–686. [Google Scholar]
- 2.Manabe, S. & Wetherald, R. T. (1986) Science 232, 626–628. [DOI] [PubMed] [Google Scholar]
- 3.Rind, D. (1988) J. Geophys. Res. 93, 5385–5412. [Google Scholar]
- 4.Valdes, J. B., Seoane, R. S. & North, G. R. (1994) J. Hydrol. 161, 389–413. [Google Scholar]
- 5.Gleick, P. H. (1989) Rev. Geophys. 27, 329–344. [Google Scholar]
- 6.Komescu, A. U., Eikan, A. & Oz, S. (1998) Climatic Change 40, 519–545. [Google Scholar]
- 7.Dickinson, R. E. (1995) Remote Sensing Environ. 51, 27–38. [Google Scholar]
- 8.Kleidon, A. & Heimann, M. (1999) Global Ecol. Biogeogr. Lett. 8, 397–405. [Google Scholar]
- 9.Schulze, E.-D., Kelliher, F. M., Körner, C., Lloyd, J. & Leuning, R. (1994) Annu. Rev. Ecol. System. 25, 629–660. [Google Scholar]
- 10.Chapin, F. S., Shaver, G. R., Giblin, A. E., Nadelhoffer, K. J. & Laundre, J. A. (1995) Ecology 76, 694–711. [Google Scholar]
- 11.Harte, J., Torn, M. S., Chang, F. R., Feifarek, B., Kinzig, A. P., Shaw, R. & Shen, K. (1995) Ecol. Appl. 5, 132–150. [Google Scholar]
- 12.Yonghui, Y., Zhiping, W., Sakura, Y., Changyuan, T. & Shindo, S. (2002) Yingyong Shengtai Xuebao 13, 667–671.12216389 [Google Scholar]
- 13.Miglietta, F., Giuntoli, A. & Bindi, M. (1996) Photosynth. Res. 47, 281–290. [DOI] [PubMed] [Google Scholar]
- 14.Topp, G. C., Davis, J. L. & Annan, A. P. (1980) Water Resourc. Res. 16, 574–582. [Google Scholar]
- 15.Price, M. V. & Waser, N. M. (1998) Ecology 79, 1261–1270. [Google Scholar]
- 16.Alatalo, J. M. & Totland, O. (1997) Global Change Biol. 3, 74–79. [Google Scholar]
- 17.Roche, C. T., Thill, D. C. & Shafii, B. (1997) Weed Sci. 45, 763–770. [Google Scholar]
- 18.Sandvik, S. M. & Totland, O. (2000) Ecoscience 7, 201–213. [Google Scholar]
- 19.Totland, O. (1997) Arctic Alpine Res. 29, 285–290. [Google Scholar]
- 20.Welker, J. M., Molau, U., Parsons, A. N., Robinson, C. H. & Wookey, P. A. (1997) Global Change Biol. 3, 61–73. [Google Scholar]
- 21.Starr, G., Oberhauer, S. F. & Pop, E. W. (2000) Global Change Biol. 6, 357–369. [Google Scholar]
- 22.Choudhury, B. J. (1987) Remote Sensing Environ. 22, 209–233. [Google Scholar]
- 23.Asrar, G., Myeni, R. B. & Kanemasu, E. T. (1989) Remote Sensing Environ. 27, 143–155. [Google Scholar]
- 24.Myeni, R. B. & Williams, D. L. (1994) Remote Sensing Environ. 49, 200–211. [Google Scholar]
- 25.Goetz, S. J. (1997) Int. J. Remote Sensing 18, 71–82. [Google Scholar]
- 26.Tieszen, L. L., Reed, B. C. & Dejong, D. D. (1997) Ecol. Apps. 7, 59–71. [Google Scholar]
- 27.Huemmrich, K. F., Black, T. A. & Hall, F. G. (1999) J. Geophys. Res. 104, 27935–27940. [Google Scholar]
- 28.Fredeen, A. L., Randerson, J. T., Holbrook, N. M. & Field, C. B. (1997) J. Am. Water Resourc. Assoc. 33, 1033–1039. [Google Scholar]
- 29.Ham, J. M. & Knapp, A. K. (1998) Agric. Forest Meteorol. 89, 1–14. [Google Scholar]
- 30.Lund, C. P. (2002) Ph.D. thesis (Stanford University, Stanford, CA), pp. 161.
- 31.Cowling, R. M., Rundel, P. W., Lamont, B. B., Arroyo, M. K. & Arianoutsou, M. (1996) Tree 11, 362–366. [DOI] [PubMed] [Google Scholar]
- 32.Groves, R. H. & DiCastri, F., eds. (1991) Biogeography of Mediterranean Invasions (Cambridge Univ. Press, Cambridge, U.K.).
- 33.Williams, K. & Hobbs, R. J. (1989) Oecologia 81, 62–66. [DOI] [PubMed] [Google Scholar]
- 34.Zavaleta, E. S., Shaw, M. R., Chiariello, N. R., Thomas, B. D., Cleland, E. E., Field, C. B. & Mooney, H. A. (2003) Ecol. Monogr., in press.