Abstract
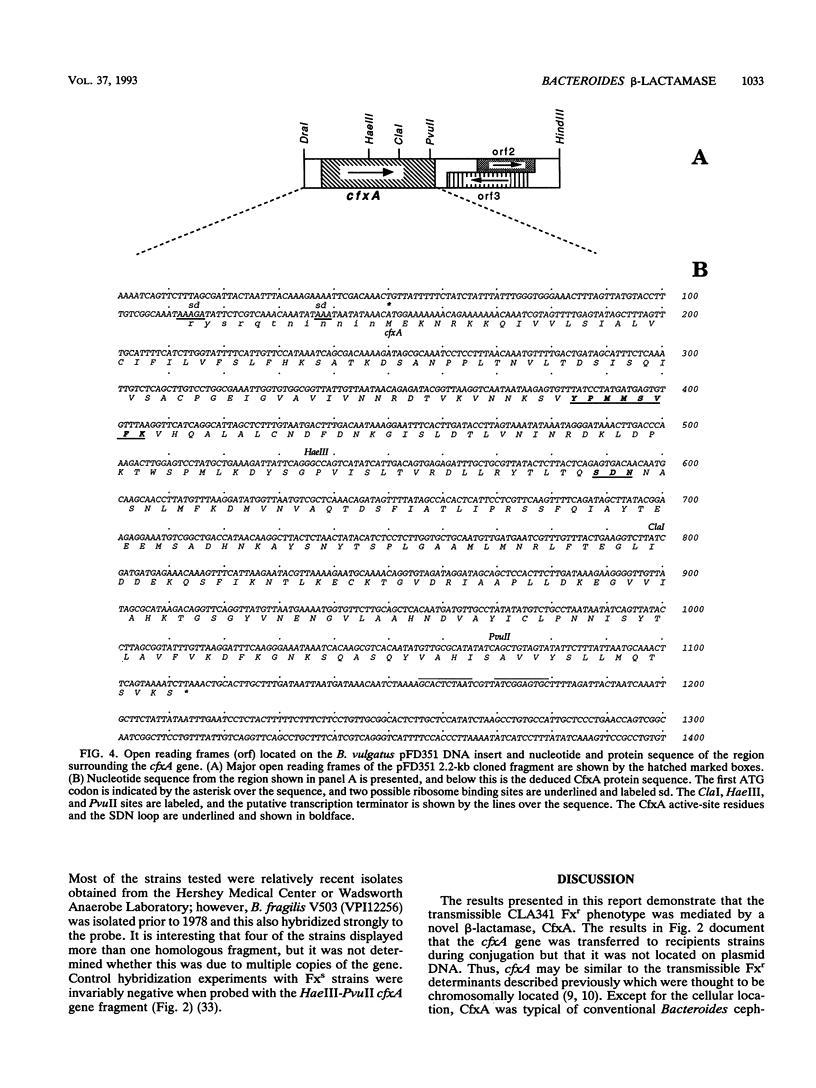
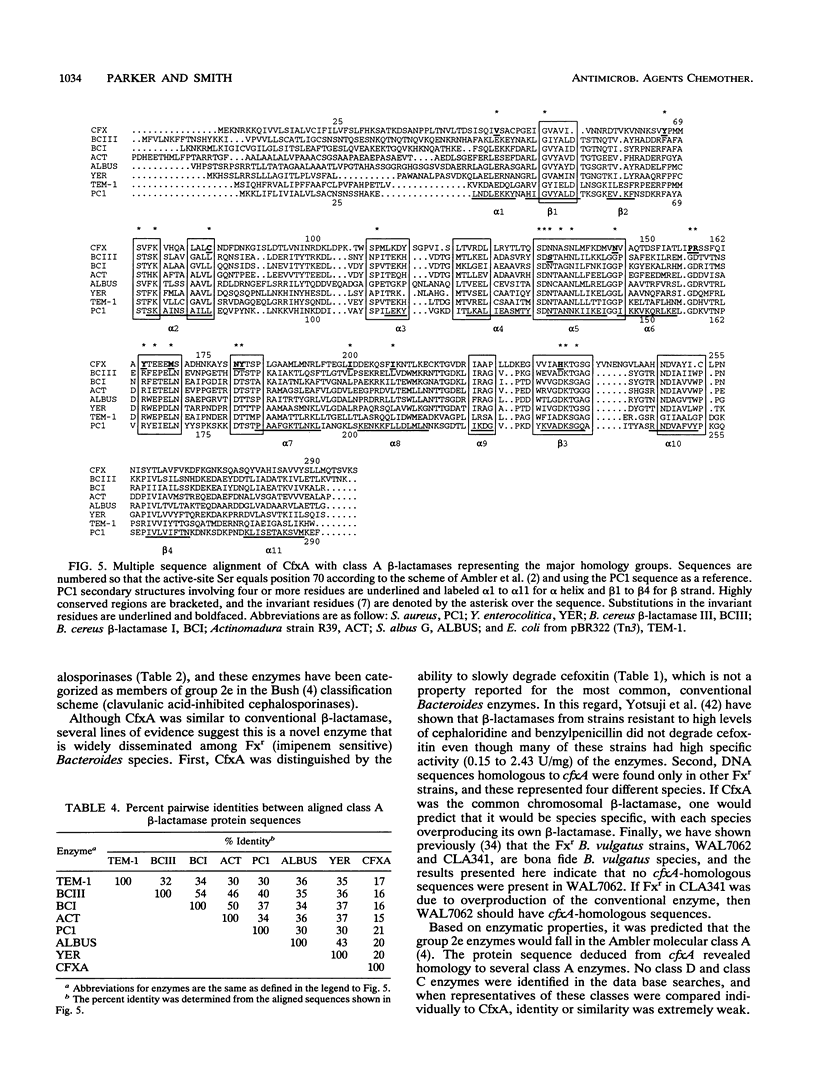
A clinical isolate of Bacteroides vulgatus was resistant to tetracycline, clindamycin, ampicillin, cephaloridine, cefoxitin, and other beta-lactam antibiotics except imipenem. beta-Lactam resistance was mediated by a membrane-associated, clavulanate-sensitive cephalosporinase capable of degrading cephalosporins and penicillins. Cefoxitin also was degraded but at a slow rate. The cefoxitin resistance (Fxr) determinant was cloned from B. vulgatus genomic libraries that were prepared in Escherichia coli and then mated with Bacteroides fragilis for the identification of Fxr strains. Analysis of B. fragilis strains with the cloned Fxr determinant revealed the presence of a new beta-lactamase protein with the physical and enzymatic properties of the beta-lactamase found in the original B. vulgatus isolate. The beta-lactamase gene (cfxA) was subcloned on a 2.2-kb DraI-HindIII fragment, and the nucleotide sequence was determined. These results showed that cfxA encoded a protein of 321 amino acids and 35,375 molecular weight. Mutant strains in which the cfxA structural gene was disrupted by insertional inactivation lost both Fxr and beta-lactamase activity. Comparison of CfxA with other beta-lactamases showed a relationship with the active-site serine beta-lactamases in the Ambler molecular class A, although CfxA had apparently diverged significantly. This was exemplified by the substitution in CfxA at 13 of 25 amino acid residues previously identified as being invariant in class A beta-lactamases. These results suggest that CfxA may represent a new class A homology group which diverged very early.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Henderberg A., Sanders C. V. In-vitro study of the susceptibility of cefoxitin/cefotetan resistant Bacteroides fragilis group strains to various other antimicrobial agents. J Antimicrob Chemother. 1990 Sep;26(3):353–359. doi: 10.1093/jac/26.3.353. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Coulson A. F., Frère J. M., Ghuysen J. M., Joris B., Forsman M., Levesque R. C., Tiraby G., Waley S. G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991 May 15;276(Pt 1):269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. T. Nucleotide sequence of the Staphylococcus aureus PC1 beta-lactamase gene. Nucleic Acids Res. 1986 Jul 25;14(14):5940–5940. doi: 10.1093/nar/14.14.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz E., Labia R., Gutmann L. Molecular evolution of ubiquitous beta-lactamases towards extended-spectrum enzymes active against newer beta-lactam antibiotics. Mol Microbiol. 1990 Oct;4(10):1615–1620. doi: 10.1111/j.1365-2958.1990.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Couture F., Lachapelle J., Levesque R. C. Phylogeny of LCR-1 and OXA-5 with class A and class D beta-lactamases. Mol Microbiol. 1992 Jun;6(12):1693–1705. doi: 10.1111/j.1365-2958.1992.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Malamy M. H., Tally F. P. Beta-lactamase-mediated imipenem resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986 Nov;30(5):645–648. doi: 10.1128/aac.30.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Marsh P. K., Mayhew J. W. Cefoxitin inactivation by Bacteroides fragilis. Antimicrob Agents Chemother. 1983 Dec;24(6):936–940. doi: 10.1128/aac.24.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Storey J. R., Malamy M. H. Transfer of beta-lactamase-associated cefoxitin resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986 May;29(5):918–920. doi: 10.1128/aac.29.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehottay P., Dusart J., De Meester F., Joris B., Van Beeumen J., Erpicum T., Frère J. M., Ghuysen J. M. Nucleotide sequence of the gene encoding the Streptomyces albus G beta-lactamase precursor. Eur J Biochem. 1987 Jul 15;166(2):345–350. doi: 10.1111/j.1432-1033.1987.tb13521.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R., Greenwood D. An investigation of beta-lactamases from clinical isolates of Bacteroides species. J Med Microbiol. 1992 Feb;36(2):89–95. doi: 10.1099/00222615-36-2-89. [DOI] [PubMed] [Google Scholar]
- Foweraker J. E., Hawkey P. M., Heritage J., Van Landuyt H. W. Novel beta-lactamase from Capnocytophaga sp. Antimicrob Agents Chemother. 1990 Aug;34(8):1501–1504. doi: 10.1128/aac.34.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E. P., Salyers A. A. Use of targeted insertional mutagenesis to determine whether chondroitin lyase II is essential for chondroitin sulfate utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1986 Jun;166(3):966–971. doi: 10.1128/jb.166.3.966-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herzberg O. Refined crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.0 A resolution. J Mol Biol. 1991 Feb 20;217(4):701–719. doi: 10.1016/0022-2836(91)90527-d. [DOI] [PubMed] [Google Scholar]
- Houba S., Willem S., Duez C., Molitor C., Dusart J., Frère J. M., Ghuysen J. M. Nucleotide sequence of the gene encoding the active-site serine beta-lactamase from Actinomadura R39. FEMS Microbiol Lett. 1989 Dec;53(3):241–246. doi: 10.1016/0378-1097(89)90224-3. [DOI] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Pastor F. I., Lampen J. O. Cloning and sequencing of the blaZ gene encoding beta-lactamase III, a lipoprotein of Bacillus cereus 569/H. J Bacteriol. 1987 Feb;169(2):579–586. doi: 10.1128/jb.169.2.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Joris B., Lepage S., Dusart J., Frère J. M. Role of the conserved amino acids of the 'SDN' loop (Ser130, Asp131 and Asn132) in a class A beta-lactamase studied by site-directed mutagenesis. Biochem J. 1990 Oct 15;271(2):399–406. doi: 10.1042/bj2710399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor N., Piñero D., Valdés A. M., Soberón X. Molecular evolution of class A beta-lactamases: phylogeny and patterns of sequence conservation. Mol Microbiol. 1990 Nov;4(11):1957–1965. doi: 10.1111/j.1365-2958.1990.tb02045.x. [DOI] [PubMed] [Google Scholar]
- Seoane A., García Lobo J. M. Nucleotide sequence of a new class A beta-lactamase gene from the chromosome of Yersinia enterocolitica: implications for the evolution of class A beta-lactamases. Mol Gen Genet. 1991 Aug;228(1-2):215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloma A., Gross M. Molecular cloning and nucleotide sequence of the type I beta-lactamase gene from Bacillus cereus. Nucleic Acids Res. 1983 Jul 25;11(14):4997–5004. doi: 10.1093/nar/11.14.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Callihan D. R. Analysis of rRNA restriction fragment length polymorphisms from Bacteroides spp. and Bacteroides fragilis isolates associated with diarrhea in humans and animals. J Clin Microbiol. 1992 Apr;30(4):806–812. doi: 10.1128/jcm.30.4.806-812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. Characterization of Bacteroides ovatus plasmid pBI136 and structure of its clindamycin resistance region. J Bacteriol. 1985 Mar;161(3):1069–1073. doi: 10.1128/jb.161.3.1069-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jacobus N. V., Gorbach S. L., Aldridge K. E., Cleary T. J., Finegold S. M., Hill G. B., Iannini P. B., McCloskey R. V. Susceptibility of the Bacteroides fragilis group in the United States in 1981. Antimicrob Agents Chemother. 1983 Apr;23(4):536–540. doi: 10.1128/aac.23.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jr Antibiotic resistance in anaerobic bacteria. J Antimicrob Chemother. 1988 Jul;22 (Suppl A):63–71. doi: 10.1093/jac/22.supplement_a.63. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jr, Jacobus N. V., Gorbach S. L., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P. Nationwide study of the susceptibility of the Bacteroides fragilis group in the United States. Antimicrob Agents Chemother. 1985 Nov;28(5):675–677. doi: 10.1128/aac.28.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Malamy M. H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus beta-lactamase II. J Bacteriol. 1990 May;172(5):2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Oyaizu Y., Oyaizu H., Woese C. R. Natural relationship between bacteroides and flavobacteria. J Bacteriol. 1985 Oct;164(1):230–236. doi: 10.1128/jb.164.1.230-236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. M., Halebian S. Alterations to the penicillin-binding proteins in the Bacteroides fragilis group: a mechanism for non-beta-lactamase mediated cefoxitin resistance. J Antimicrob Chemother. 1990 Jul;26(1):7–20. doi: 10.1093/jac/26.1.7. [DOI] [PubMed] [Google Scholar]
- Yotsuji A., Minami S., Kakizawa H., Yasuda T., Takai A., Saikawa I., Inoue M., Mitsuhashi S. Cephamycin inactivation due to enzymatic hydrolysis by beta-lactamase from Bacteroides fragilis. Antimicrob Agents Chemother. 1985 Dec;28(6):773–777. doi: 10.1128/aac.28.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]