Abstract
Mating induces profound changes in female insect behavior and physiology. In Drosophila melanogaster, mating causes a reduction in sexual receptivity and an elevation in egg production for at least 5 days. Injection of the seminal fluid sex peptide (SP) induces both responses in virgin females, but only for 1–2 days. The role of SP in eliciting the responses to mating remains to be elucidated. Functional redundancy between seminal fluid components may occur. In addition, mating with spermless males results in brief (1- to 2-day) post-mating responses, indicating either that there is a “sperm effect” or that sperm act as carriers for SP or other seminal fluid components. Here we used RNA interference to suppress SP expression, to determine whether SP is required to elicit full post-mating responses, the magnitude of responses due to other seminal fluid components, and whether SP accounts for the “sperm effect.” Receptivity was higher and egg production lower in females mated to SP knock-down males than in controls. Comparison with virgins showed that the responses were brief. SP is therefore required for normal magnitude and persistence of postmating responses. Sperm transfer and use were normal in mates of SP knock-down males, yet their post-mating responses were briefer than after normal matings, and similar to those reported in mates of spermless son-of-tudor males. The prolonged “sperm effect” on female receptivity and egg production is therefore entirely attributable to SP, but sperm are necessary for its occurrence.
In many insects, mating induces striking changes in the behavior and physiology of females (1, 2). These post-mating responses of females are of interest because of their potential utility in insect pest control (3, 4) and because they appear to be subject to unusually strong natural selection (5–9). After mating, female insects can become temporarily unattractive, they can show reduced receptivity to mating, and they can increase their rate of egg production (e.g., refs. 10–17). In Drosophila melanogaster females, post-mating responses are elicited by pheromones, sperm, and male ejaculate proteins (reviewed in refs. 18 and 19). The accessory glands of the male were originally implicated in inducing post-mating responses of D. melanogaster females because transplantation of whole glands and injection of gland extracts into females increased egg production and reduced receptivity (13, 20, 21). Injection of HPLC fractions of accessory gland extracts led to the isolation of the 36-aa sex peptide (SP or Acp70A). Injection of physiological amounts of purified or synthetic SP caused virgin females to become unreceptive to mating and stimulated egg production, for a period of 1–2 days (22). Transfer of SP may, therefore, be at least in part responsible for the reduced receptivity and increased egg production in D. melanogaster females after mating. However, the role of SP after a normal mating remains to be elucidated.
Although SP injection affects female receptivity and egg laying, SP may not be necessary at mating for these responses to occur. Functional redundancy between SP and other accessory fluid proteins (Acps) in determining post-mating receptivity and egg production could involve at least two other ejaculate components: Acp26Aa (ovulin) and Dup99B (23, 24). Females mated to males that are null for the Acp26Aa gene produce ≈18% fewer eggs on the first day after mating, and ovulate at a far lower rate, than do mates of wild-type mates (23, 25). Dup99B, produced in the ejaculatory duct of the male, and the SP exhibit strong sequence similarity in their C-terminal regions, and the genes that encode them may have arisen by gene duplication (24). Injection of purified Dup99B causes SP-like effects on female receptivity and egg production (24). However, females mated to males that transfer Dup99B but no SP or other Acps show no or very brief (1- to 2-h) reduction in receptivity (24, 26, 27) and only a slight increase in egg production (27) and none in the absence of sperm (26). Other, as-yet-unidentified, male-derived factors may also affect subsequent female receptivity and rate of egg production. Matings with males that lack the SP are necessary to determine its unique role in female post-mating responses.
SP may or may not interact with sperm in its effects on receptivity and egg production. After a normal mating, both responses persist for at least 5 days (28), rather than the 1–2 days seen after SP injection or after mating with males that do not transfer sperm (26–29). The more rapid return of female receptivity and egg laying to virgin levels after a spermless mating led to the idea of a “sperm effect” (28). Alternatively, because SP binds to sperm, sperm may be necessary at mating for the response to SP to exceed 1–2 days (24). A further possibility is that an interaction between sperm and some other seminal fluid component is responsible for the “sperm effect.” Matings with males that lack SP but that transfer sperm are needed to elucidate the role of SP in the sperm effect.
Examination of genetic variation within and between species has shown that Acps evolve rapidly as a result of strong natural selection (5, 7–9). To understand this rapid evolution, it is necessary to elucidate the role of Acps in determining female and male reproductive success. Acps could enhance offspring production of both sexes if, for instance, they caused egg production to be stimulated and coordinated only after mating, when sperm are available for fertilization. Seminal fluid proteins could enhance male reproductive success only. For instance, particular Acps are essential for normal sperm storage (30) and success in sperm competition (31). Acps can increase male success in competition with other males, for instance by disabling or removing the sperm of previous mates (32) or by delaying the interval until the female mates again (22), thus increasing the time for which eggs are fertilized by the sperm of the first male (32–34). Acps can also increase male reproductive success at the expense of that of females. As-yet-unidentified Acps (although see ref. 35) can increase mortality rate of females and hence reduce their lifetime reproductive success, presumably as an unselected side-effect of male-beneficial functions of the same Acps (ref. 33, but see ref. 36). The role of Acps in competition between males for mating and in sexual conflict between males and females may account for their rapid evolutionary change under selection (5–9). To determine the way that selection acts on Acps, it is necessary to determine the effects of their removal on female and male reproductive success.
We have produced males that lack detectable SP in their seminal fluid by using RNA interference (RNAi) (37). RNAi is a powerful technique for inducing targeted suppression of gene expression in D. melanogaster (e.g., ref. 38). We produced three independent lines carrying an SP sense-antisense transgene, and drove the expression of this construct in the normal site of expression for the SP gene, the male accessory glands. Females mated to these SP knock-down males were significantly more willing to remate and had significantly lower ovulation and egg production than mates of control males. SP is thus necessary for normal expression of these post-mating responses. Mates of SP knock-down males initially showed significantly lower receptivity and higher egg production than virgin females, but these responses were of smaller magnitude than normal, particularly for receptivity. Other Acps, or Acp interactions with sperm, must produce these additional short-term post-mating effects. Sperm transfer and use was normal in SP knock-down males. Despite this, the responses of females mated to knock-down males were similar to those reported for mates of males that transfer full ejaculate components but no sperm [son-of-tudor males (26, 27)]. This observation suggests that sperm are necessary for full SP activity after mating, and that the sperm effect (28) is in fact an SP effect.
Materials and Methods
Wild-Type Strain and Fly Culturing. Wild-type flies were from a stock collected in Dahomey (now Benin) in 1970. All experiments were conducted at 25°C on a 12/12-h light/dark cycle. Maize-yeast medium [10 g of agar, 85 g of sugar, 60 g of maize, 20 g of autolyzed yeast, and 25 ml of Nipagin (methyl 4-hydroxybenzoate) per 1,000 ml of water] supplemented with live yeast was used throughout. Flies were reared in vials (23 mm by 73 mm) containing 7 ml of medium. Wild-type experimental flies were obtained by placing first-instar larvae in groups of 50 each into vials. Virgin flies were collected from these standard density cultures within 7 h of eclosion.
Generation of SP RNAi and Gal4 Driver Transgenic Stocks. SP knock-down males were produced by generating flies with SP RNAi transgenes. We produced stocks with a pUAST transgene (vector donated by Andrea Brand, University of Cambridge, Cambridge, U.K.) with UAS (upstream activating sequence) upstream of a 305-bp portion of the SP gene in the sense-antisense orientation. A 305-bp fragment covering the entire SP coding region was amplified from genomic DNA (forward primer GAAGATCTGGTGTAAAATGAAAACTCTAGC, reverse CGGGATCCGATTTTAAGACATTTTGGTGGG). Amplified SP sequence was digested with BglII and cloned between the BglII site and the (blunted) XhoI site in pUAST to give a vector, named pSPsense, carrying a single copy of the SP gene. Amplified SP sequence, digested with BglII and BamHI, was cloned into pSPsense that had been digested with BglII and treated with calf intestine alkaline phosphatase, using the Escherichia coli SURE strain (Stratagene). The resulting vector, named pSP-IR, was marked with w+, and carried two copies of the SP sequence in an inverted repeat orientation. Clone structure of pSPsense and pSP-IR was verified by multiple restriction digestions. Transcription of this transgene is predicted to produce a hairpin loop with sense 5′ to the antisense sequence.
pSP-IR transgenic flies were constructed as previously described (39, 40) by injection of pSP-IR into a w1 genetic background. Homozygous viable and fertile stocks were obtained by backcrossing w+ individuals to the w1 injection stock and then crossing w+ individuals among themselves. Thus the genetic background of the lines remained purely that of the injection stock. Balancer chromosomes were subsequently used purely for mapping the inserts.
An accessory-gland-specific Gal4 driver line was established by generating transgenic flies carrying the Acp26Aa promoter [≈1.4 kb upstream of the coding region (41) fused to Gal4, or Acp26Aa-P-Gal4]. The construct was injected into a z1w11e4 background, and transgenic individuals were recovered as w+ progeny. A homozygous viable stock was obtained by crossing transgenic individuals to the z1w11e4 background used for injections, followed by crossing the progeny among themselves. We confirmed that this construct drives accessory gland-specific expression of a UAS-transgene by staining reproductive tracts of 3-day-old virgin males (Acp26Aa-P-Gal4;UAS-lacZ or homozygous UAS-lacZ controls) for β-galactosidase (LacZ) by using minor modifications of the method in ref. 42.
Western Blotting. The level of SP in males carrying the Acp26Aa-P-Gal4 and UAS-SP-IR (inverted repeat) constructs was determined by Western blotting. Putative SP knock-down males were obtained by crossing homozygous males from each of the three inverted repeat lines obtained (UAS-SP-IR1, UAS-SP-IR2, and UAS-SP-IR3) to virgin females from the Acp26Aa-P-Gal4 line. The resulting males (Acp26Aa-P-Gal4;UAS-SP-IR1, Acp26Aa-P-Gal4;UAS-SP-IR2, and Acp26Aa-P-Gal4;UAS-SP-IR3) were analyzed. Positive controls were the male offspring of the reciprocal crosses, i.e., homozygous females from each of the three UAS-SP-IR lines crossed to Acp26Aa-P-Gal4 males. The resulting control males (+;UAS-SP-IR1, +;UAS-SP-IR2, and +;UAS-SP-IR3) shared the genetic background of the putative knock-down males, except for the X chromosome. Additional controls were homozygous males from each UAS-SP-IR insert line and homozygous Acp26Aa-P-Gal4 males.
We used young, mated males because optimization experiments predicted they would have maximal SP knock-down. Quantification of Acp expression in flies carrying an Acp62F-IR RNAi transgene and the Acp26Aa-Gal4 driver showed that young mated males had increased knock-down (to <3% of control levels, O.L. and M.F.W., unpublished data) relative to older, or unmated, males. This finding is likely to be due to the combined effects of mating on the depletion of existing Acps (by 75% in 1-day-old males, O.L. and M.F.W. unpublished data), and mating-induced transcription from the Acp26Aa promoter (43) used in the driver construct. Levels of Acps 26Aa and 36DE were unaffected by RNAi of Acp62F, verifying that knock-down by this method is Acp-specific.
Two-day old virgin male progeny from all experimental and control crosses were mated en masse to females from their own lines. These mated males, and virgin males of the same age, were then transferred in groups of 20 to fresh vials. Twenty-four hours later, groups of five males were transferred into chilled Eppendorf tubes with 40 μl of homogenization buffer (50 mM Tris·HCl, pH 7.5/10 mM EDTA, pH 8) and partially homogenized. Forty microliters of 2× sample buffer [125 mM Tris·HCl, pH 6.8/20% (vol/vol) glycerol/4% SDS/0.01% bromophenol blue/10% (vol/vol) 2-mercaptoethanol] was then added, and the samples were fully homogenized, boiled for 4 min, transferred to ice for 2 min, centrifuged at 100 × g for 5 min at 4°C, and snap-frozen in liquid N2. An equal amount of protein extract for each line was loaded on an SDS/polyacrylamide gel (15% acrylamide/bisacrylamide) and subjected to electrophoresis at 120 V for ≈1 h. The gel was blotted with Towbin buffer on Hybond ECL nitrocellulose membrane (Amersham Pharmacia). The membrane was washed in blocking solution [5% low-fat dry milk in PBS/0.1% Tween 20 (PBS-T)] for 1 h and incubated for 1.5 h with the primary antibody (anti-SP rabbit antibodies, donated by Eric Kubli, University of Zurich-Irchel, Zurich). After washing with PBS-T solution, the membrane was incubated with peroxidase-labeled anti-rabbit secondary antibody (Amersham Pharmacia) for 1 h, then treated with the ECL Western blotting detection system (Amersham Pharmacia), according to the manufacturer's instructions.
Real-Time PCR. We also measured the levels of SP and Dup99B RNA in Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2 males and their controls (males were obtained as described above). Primers were designed by using Applied Biosystems Prism Primer Express Version 2.0 (SP forward primer GAATGGCCGTGGAATAGGAA, reverse GGCACCACTTATCACGAGGATT; Dup99B forward CGCTATTTCTCCTCTTGGTCGTA, reverse TCTCACGATCCTTCTGACTTTGG). We used the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) for cDNA synthesis. Real-time PCR was performed by using an Applied Biosystems Prism 7000 Sequence Detection System, and Sybr green (Molecular Probes), ROX Reference Dye (Invitrogen), and HotStar Taq DNA polymerase (Qiagen, Valencia, CA). Quantification of transcript levels in SP knock-down males relative to their controls (all normalized to actin 5C) was performed by using a standard curve method (following Applied Biosystems protocols).
Generation of Experimental Males. For the receptivity, ovulation, and oviposition assays, two lines of males carrying the SP inverted repeat and Gal4 transgenes (Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2) were used. The third SP knock-down line (UAS-SP-IR3) was not used because, although like the first two lines it produced knock-down levels of SP, the insert appeared to be unstable. We worked with two matched pairs of experimental and control lines: +;UAS-SP-IR1 and +;UAS-SP-IR2 males acted as controls for Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2 males, respectively. Five virgin male and five virgin female parents of each cross were placed into vials for 3 days and transferred into fresh vials for a further 3 days. Virgin male offspring were collected from these vials over 4 days. For all experimental and control crosses, 1- to 3-day-old virgin male offspring were mated en masse to females from their own vials and stored in single-sex groups of 10 for 1–2 days before use in the experiments.
Receptivity Assay. To determine the effect of the SP on female receptivity, wild-type females were mated once to SP knock-down or control males, then exposed to wild-type males in a receptivity assay 24 and 48 h later. Virgin wild-type females were collected at eclosion and stored 10 per vial for 2 days. Pairs consisting of a single 4- to 5-day-old knock-down or control male, and a single 4-day-old wild-type virgin female, were then put into individual vials. Approximately 130 pairs of each cross and control were set up. Pairs were observed for about 8 h and 120–150 pairs from each line were mated. Immediately after mating, males were removed. After 24 h, a 5-day-old wild-type male was introduced into each vial and the number of females remating within 1 h was recorded. This test was repeated 48 h after the initial matings, with 6-day-old wild-type males.
Oviposition Assay. Two-day-old wild-type virgin females were mated in single pairs to 4- to 5-day-old SP knock-down or control males. Sixty pairs of each cross and control were set up. Females were then transferred to fresh vials every 24 h for 5 days and eggs laid every day were counted.
Ovulation Assay. Three-day-old virgin wild-type females were mated in single pairs to 5- to 6-day-old SP knock-down or control males. Seventy pairs of each cross and control were set up. At 6, 24, and 48 h after the first mating, subsets of ≈25 females from each group were dissected in PBS to determine the percentage of females in each group with an egg in the uterus (5).
Egg Fertility Assay. Vials from days 1, 3, and 5 after mating in the oviposition assay described above were retained to count progeny to determine percentage egg fertility [(number of pupae/number of eggs laid) × 100].
Results and Discussion
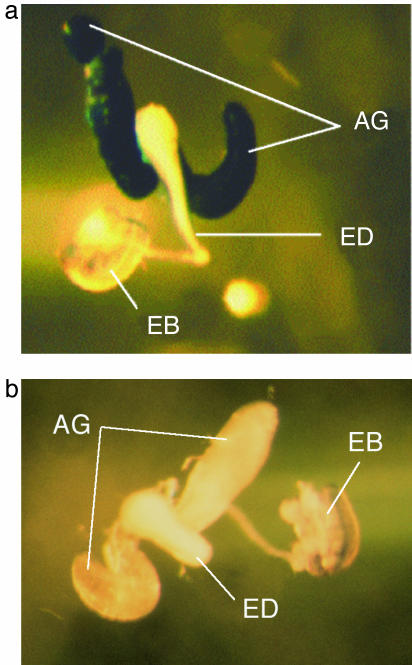
Recovery of SP RNAi and Gal4 Transgenic Stocks. Three independent SP-RNAi transgenic lines were recovered, UAS-SR-IR1, UAS-SP-IR2, and UAS-SP-IR3, with insertions on chromosomes III, II, and II, respectively. The UAS-SP-IR3 insertion appeared to be unstable, as w1 revertants were sometimes observed in the stock. For this reason, experiments were conducted with only two of the transgenic stocks (i.e., UAS-SP-IR1 and UAS-SP-IR2). For the Acp26Aa-P-Gal4 driver line, one insert was recovered and mapped to the X chromosome. To confirm that the transgene drives accessory-gland-specific expression, virgin transgenic females were crossed to males homozygous for UAS-lacZ, and the accessory glands of the adult male progeny were stained for LacZ expression (Fig. 1). LacZ expression was seen in the accessory glands of Acp26Aa-P-Gal4;UAS-lacZ males (Fig. 1a) but not homozygous UAS-lacZ control males (Fig. 1b).
Fig. 1.
Specificity of expression driven by the Acp26Aa-P-Gal4 transgene, demonstrated by LacZ expression in Acp26Aa-P-Gal4;UAS-lacZ males. (a) Acp26Aa-P-Gal4 drives expression of UAS-lacZ in male accessory glands (AG) but not the ejaculatory duct (ED) or ejaculatory bulb (EB). (b) No LacZ expression in is detected in control males, homozygous for UAS-lacZ but lacking the driver.
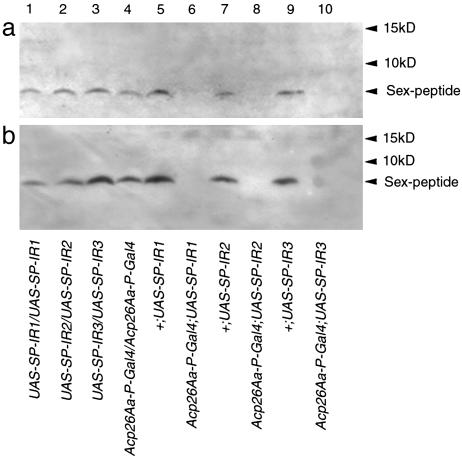
SP Is Undetectable After RNAi. Western blotting (Fig. 2) showed that males carrying the Acp26Aa-P-Gal4 driver and any of the three UAS-SP-IR transgenes produced no detectable SP. All other control males produced detectable SP. RNAi activated specifically in the male accessory glands knocked down the SP to levels that were not detectable by Western analysis, in both 3-day-old SP knock-down virgin males (Fig. 2a) and 3-day-old SP knock-down males mated when 2 days old (Fig. 2b). SP RNA levels in SP knock-down males were strongly reduced (10.5% and 1.2% of control levels for Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2 males, respectively). In contrast, the level of Dup99B RNA in SP knock-down males was high (94.9% and 74.1% of control levels for Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2 males, respectively). Results were averaged across two RNA extractions.
Fig. 2.
Western blot showing levels of SP in SP knock-down and control males in 3-day-old virgin males (a) and in 3-day-old males mated when 2 days old (b). From left, in lanes 1–4, homozygous UAS-SP-IR1, UAS-SP-IR2, UAS-SP-IR3, and Acp26Aa-P-Gal4 control males produced SP [molecular mass 4,428 Da (22)]. In lanes 5, 7, and 9, control males carrying the inverted repeat insert without the Gal4 driver (+;UAS-SP-IR1, +;UAS-SP-IR2, and +;UAS-SP-IR3) produced SP. In lanes 6, 8, and 10, males with the inverted repeat and the Gal4 driver (Acp26Aa-P-Gal4;UAS-SP-IR1, Acp26Aa-P-Gal4;UAS-SP-IR2, and Acp26Aa-P-Gal4;UAS-SP-IR3) produced no detectable SP.
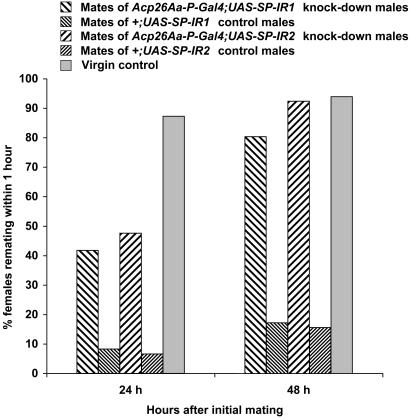
Effect of SP Knock-Down on Female Receptivity. The receptivity of females mated to SP knock-down males (Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2) was compared with females mated with the respective controls (+;UAS-SP-IR1 and +;UAS-SP-IR2) at 24 and 48 h after their first matings (Fig. 3). Females that mated with males deficient in the SP were significantly more receptive than were females mated to control males. At 24 h the receptivity of females mated to SP knock-down males was intermediate between that of females mated to control males and that of virgin females. By 48 h the receptivity of females mated to SP knock-down males was similar to that of virgins. Females mated to SP knock-down males therefore did not behave like virgin females in terms of receptivity until 48 h after mating; there was some residual reduction in receptivity caused by matings to SP knock-down males.
Fig. 3.
Effect of SP on female receptivity. Shown is percentage of females remating within1hina receptivity test with wild-type males, 24 and 48 h after initial matings to Acp26Aa-P-Gal4;UAS-SP-IR1 SP knock-down males, +;UAS-SP-IR1 control males, Acp26Aa-P-Gal4;UAS-SP-IR2 SP knock-down males, or +;UAS-SP-IR2 control males. The receptivity of virgin females is also shown. The numbers of females that did and did not mate after each type of initial mating were analyzed in 2 × 2 contingency tables by using Fisher exact tests. Females mated to SP knock-down males (Acp26Aa-P-Gal4;UAS-SP-IR1 and Acp26Aa-P-Gal4;UAS-SP-IR2) were significantly more receptive than their respective controls (i.e., mates of +;UAS-SP-IR1 and +;UAS-SR-IR2 males), P < 0.0001, all tests. Females mated to both lines of SP knock-down males were significantly less receptive than virgin females at 24 h (P < 0.0001, both comparisons). At 48 h, females initially mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males were only marginally less receptive than virgins (P = 0.03), and females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males did not differ significantly in receptivity compared with virgin females.
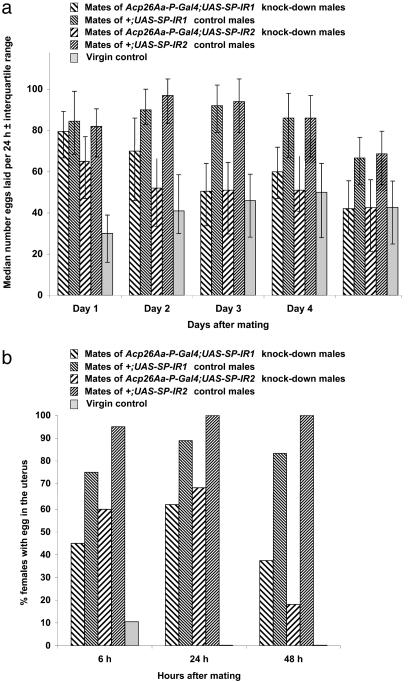
Effect of SP Knock-Down on Oviposition and Ovulation. On each of the 5 successive days after mating, females mated to SP knock-down males laid significantly (with one exception) fewer eggs than did females mated to control males (Fig. 4a). On days 1–2, females mated to SP knock-down males laid eggs at a level intermediate between that of females mated to control males and that of virgin females. Thereafter, the number of eggs deposited by mates of the SP knock-down males was similar to that of virgins (Fig. 4a). At 6, 24, and 48 h after mating, females mated to SP knock-down males from both lines showed significantly (with one exception) lower ovulation than mates of control males, and significantly (with one exception) higher ovulation than virgin females (Fig. 4b). Thus mates of SP knock-down males did not show a full mated response and their egg laying dropped down again to virgin levels 2–3 days after mating.
Fig. 4.
Effect of SP on oviposition and ovulation. (a) The median (± interquartile range) numbers of eggs laid per 24 h by females over 5 days, after mating to SP knock-down or control males. Data for unmated virgin females are also shown. The data were analyzed by using Wilcoxon tests. Females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 SP knock-down males did not differ at 24 h in egg production from mates of their respective controls (mates of +;UAS-SP-IR1 males), but laid significantly fewer eggs than their mated controls on days 2–5 (P < 0.0001, all comparisons). Females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males laid significantly fewer eggs than their controls (mates of +;UAS-SP-IR2 males) on all days (P < 0.0001, all comparisons). Females mated to both SP knock-down line males produced significantly more eggs than virgin females on the first day after mating (P < 0.001, both tests). Females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males also produced significantly more eggs than virgin females on day 2 after mating (P < 0.0001), but the egg production of these females then became similar to that of virgin females on days 3 (P = 0.3), 4 (P = 0.02), and 5 (P = 0.7). Egg production of females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males was not significantly different from that of virgins on days 2–5(P > 0.1, all tests). (b) The percentage of females with a egg in the uterus 6, 24, and 48 h after mating to SP knock-down or control males. Data for virgin females are also shown. The numbers of females that did and did not have an egg in the uterus were analyzed in 2 × 2 contingency tables by using Fisher exact tests. Females mated to SP knock-down males were significantly less likely to have an egg in the uterus than were their respective control females (females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males versus their controls, at 6 h P = 0.1, at 24 h P = 0.03, at 48 h P = 0.0003; females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males versus their controls, at 6 h P = 0.02, at 24 h P = 0.003, at 48 h P < 0.0001). Females mated to SP knock-down males were also significantly more likely (in all comparisons except one) to have an egg in the uterus than were virgin females (females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males versus virgins, at 6 h P = 0.03, at 24 h P < 0.0001, at 48 h P = 0.002; females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males versus virgins, at 6h P = 0.002, at 24 h P < 0.0001, at 48 h P = 0.1).
The egg production and ovulation tests showed that females mated to males deficient in the SP produced significantly fewer eggs than females mated to control males for the 5 days after mating. However, in the first 1–2 days after matings to SP knock-down males, females did not behave like virgins, although their egg production did become comparable to that of virgins after 2–3 days. Thus some residual stimulation of egg production is achieved after matings to SP knock-down males. This stimulation of egg production and ovulation is presumably caused by the transfer of other ejaculate proteins, such as Acp26Aa and Dup99B.
Effect of SP Knock-Down on Egg Fertility. There were no significant differences in egg fertility in mates of SP knock-down and control males on days 1 and 3 after mating (Fig. 5). On day 5 the egg fertility of mates of control males was significantly lower than of mates of SP knock-down males (Fig. 5). Egg production was significantly higher in the control females, which would have led them to run out of sperm more quickly (44) than females mated to SP knock-down males. On day 5, all females laid equal numbers of fertile eggs, suggesting that there were no significant differences in the numbers of sperm stored across treatments. The results show that SP knock-down males transferred sperm and that these sperm were stored and used in numbers comparable to those of control males.
Fig. 5.
Effect of SP on egg fertility. Egg fertility [(number of pupae/number of eggs laid) × 100, ± interquartile range] of females 1, 3, and 5 days after mating to SP knock-down or control males. The data were analyzed by using Wilcoxon tests. On days 1 and 3, the fertility of eggs produced by females mated to SP knock-down males was not significantly different from the fertility of eggs produced by females mated to control males (females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males versus their controls, on day 1 P = 0.2, on day 3 P = 0.05; females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males versus their controls, on day 1 P = 0.5, on day 3 P = 0.2). On day 5, the fertility of eggs laid by control females was significantly lower than that of females mated to SP knock-down males (females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males versus their controls, P < 0.0001, females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males versus their controls, P = 0.0007). On day 5, females of all lines produced nonsignificantly different numbers of fertile eggs (females mated to Acp26Aa-P-Gal4;UAS-SP-IR1 males versus their controls, P = 0.8, females mated to Acp26Aa-P-Gal4;UAS-SP-IR2 males versus their controls, P = 0.6).
Conclusions
The results confirm that SP is necessary for some post-mating responses of females. We used two matched pairs of experimental and control lines, and the consistent findings with them indicate that the effects on the post-mating responses were attributable to the absence of the SP, rather than to some other effect of genetic background. Females mated to SP knock-down males produced by RNAi were significantly more receptive and laid and ovulated significantly fewer eggs than did mates of control males. RNAi has therefore proved to be a powerful technique for the in vivo characterization of SP function. There was some residual reduction in receptivity and stimulation of egg production in the mates of SP knock-down males. We conclude that these residual effects in mates of SP knock-down males are due to ejaculate components and not to pheromone transfer or mating itself, because mates of DTA-E males (which mate and transfer pheromones but no Acps or sperm) show virgin levels of egg production and receptivity (26). The residual effects in mates of SP knock-down males were presumably due at least in part to as-yet-unidentified ejaculate component(s), because the other molecules so far shown to mediate these effects have smaller and/or shorter-lived effects (Dup99B), or affect only egg production and not receptivity (Acp26Aa).
Our results are quantitatively similar to those of Liu and Kubli (45), who analyzed the responses of females mated to SP gene knockout males produced by homologous recombination. Mates of SP knockout males also showed some residual reduction in receptivity and stimulation of egg production. The responses were smaller than those observed in the present study, possibly attributable to differences in the fly strains used, or differences in the fly food or culturing techniques. For instance, the rate of egg laying by virgin females differed in the two studies. This trait shows substantial natural genetic variation between strains as well as clinal geographic variation (46).
Sperm transfer and use appeared normal in matings with SP knock-down males, because egg fertility was unimpaired. Despite the presence of sperm, females mated to SP knock-down males showed post-mating responses similar to those of mates of son-of-tudor males, which transfer Acps and other ejaculate proteins but no sperm (26, 27). These findings show that the sperm effect is in fact an effect of SP, but one that is manifest only in the presence of sperm. Sperm may act as carriers of SP, with slow release prolonging the SP response.
Our results suggest SP is unlikely to cause the increased mortality in females that is attributable to as-yet-unidentified Acps (33). There is no reduction in the cost of mating in mates of son-of-tudor males, which do not transfer sperm, compared with mates of wild-type males (47). Therefore, because sperm are necessary for full SP transfer, SP is unlikely to be responsible for the Acp-mediated cost of mating in females (33). Further work with these SP knock-down males is necessary to confirm this hypothesis, and to determine the net effect of SP on male and female reproductive success.
Acknowledgments
We thank Stuart Wigby, Ken Howard, Camilla Burnett, Steven Goodall, Susan Broughton, Uyen Tram, and Michael Silverman for help with experiments; and Eric Kubli for helpful comments on the manuscript and donation of the SP antibody. Funding was provided by the Royal Society (university research fellowship to T.C.), the Biotechnology and Biological Sciences Research Council (Ph.D. studentship to J.B. and professorial fellowship to L.P.), University College London (funding for G.V. and B.S.), the Wellcome Trust (equipment), and National Institutes of Health Grant HD38921 (to M.F.W.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SP, sex peptide; Acps, accessory fluid proteins; RNAi, RNA interference.
See commentary on page 9643.
References
- 1.Chen, P. S. (1984) Annu. Rev. Entomol. 29, 233–255. [Google Scholar]
- 2.Gillot, C. (2003) Annu. Rev. Entomol. 48, 163–184. [DOI] [PubMed] [Google Scholar]
- 3.Miller, J. R., Spencer, J. L., Lentz, A. J., Keller, J. E., Walker, E. D. & Leykam, J. F. (1994) ACS Symp. Ser. 551, 189–209. [Google Scholar]
- 4.Partridge, L. (1996) in Fruit Fly Pests: A World Assessment of Their Biology and Management, eds. McPheron, B. A. & Steck, G. J. (St. Lucie Press, Boca Raton, FL), pp. 9–15.
- 5.Aguadè, M., Miyashita, N. & Langley, C. H. (1992) Genetics 132, 755–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice, W. R. & Holland, B. (1997) Behav. Ecol. Sociobiol. 41, 1–10. [Google Scholar]
- 7.Tsaur, S. C. & Wu, C. I. (1997) Mol. Biol. Evol. 14, 544–549. [DOI] [PubMed] [Google Scholar]
- 8.Begun, D. J., Whitley, P., Todd, B. L., Waldrip-Dail, H. M. & Clark, A. G. (2000) Genetics 156, 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson, W. J., Clark, A. G., Waldrip-Dail, H. M., Wolfner, M. F. & Aquadro, C. F. (2001) Proc. Natl. Acad. Sci. USA 98, 7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, G. B. (1967) Science 156, 1499–1501. [DOI] [PubMed] [Google Scholar]
- 11.Nelson, D. R., Adams, T. S. & Pomonis, J. G. (1969) J. Econ. Entomol. 62, 634–639. [Google Scholar]
- 12.Riemann, J. G. & Thorson, B. J. (1969) Ann. Entomol. Soc. Am. 62, 828–834. [DOI] [PubMed] [Google Scholar]
- 13.Chen, P. S. & Bühler, R. (1970) J. Insect Physiol. 16, 615–627. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, M. S. & Hiss, E. A. (1970) J. Insect Physiol. 16, 931–939. [DOI] [PubMed] [Google Scholar]
- 15.Baumann, H. (1974) J. Insect Physiol. 20, 2347–2362. [DOI] [PubMed] [Google Scholar]
- 16.Gillot, C. & Langley, P. A. (1981) Physiol. Entomol. 6, 269–281. [Google Scholar]
- 17.Spencer, J. L., Bush, G. L., Keller, J. E. & Miller, J. R. (1992) J. Insect Behav. 5, 689–697. [Google Scholar]
- 18.Wolfner, M. F. (1997) Insect Biochem. Mol. Biol. 27, 179–192. [DOI] [PubMed] [Google Scholar]
- 19.Chapman, T. (2001) Heredity 87, 511–521. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Bellido, A. (1964) Z. Naturforsch. 19b, 491–495. [PubMed] [Google Scholar]
- 21.Leahy, M. G. & Lowe, M. L. (1967) Life Sci. 6, 151–156. [PubMed] [Google Scholar]
- 22.Chen, P. S., Stumm-Zollinger, E., Aigaki, T., Balmer, J., Bienz, M. & Böhlen, P. (1988) Cell 54, 291–298. [DOI] [PubMed] [Google Scholar]
- 23.Herndon, L. A. & Wolfner, M. F. (1995) Proc. Natl. Acad. Sci. USA 92, 10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saudan, P., Hauck, K., Soller, M., Choffat, Y., Ottiger, M., Sporri, M., Ding, Z. B., Hess, D., Gehrig, P. M., Klauser, S., et al. (2002) Eur. J. Biochem. 269, 989–997. [DOI] [PubMed] [Google Scholar]
- 25.Heifetz, Y., Lung, O., Frongillo, E. A. & Wolfner, M. F. (2000) Curr. Biol. 10, 99–102. [DOI] [PubMed] [Google Scholar]
- 26.Kalb, J. M., Dibenedetto, A. J. & Wolfner, M. F. (1993) Proc. Natl. Acad. Sci. USA 90, 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue, L. & Noll, M. (2000) Proc. Natl. Acad. Sci. USA 97, 3272–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning, A. (1962) Nature 194, 252–253. [Google Scholar]
- 29.Hihara, F. (1981) Zool. Mag. 90, 307–316. [Google Scholar]
- 30.Neubaum, D. M. & Wolfner, M. F. (1999) Genetics 153, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman, T., Neubaum, D. M., Wolfner, M. F. & Partridge, L. (2000) Proc. R. Soc. London Ser. B 267, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harshman, L. G. & Prout, T. (1994) Evolution 48, 758–766. [DOI] [PubMed] [Google Scholar]
- 33.Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. (1995) Nature 373, 241–244. [DOI] [PubMed] [Google Scholar]
- 34.Chapman, T. & Partridge, L. (1996) Nature 381, 189–190. [DOI] [PubMed] [Google Scholar]
- 35.Lung, O., Tram, U., Finnerty, C. M., Eipper-Mains, M., Kalb, J. M. & Wolfner, M. F. (2002) Genetics 160, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone, R. A. & Keller, L. (2000) Amer. Nat. 156, 368–377. [DOI] [PubMed] [Google Scholar]
- 37.Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E. & Mello, C. C. (1998) Nature 391, 806–811. [DOI] [PubMed] [Google Scholar]
- 38.Kennerdell, J. R. & Carthew, R. W. (1998) Cell 95, 1017–1026. [DOI] [PubMed] [Google Scholar]
- 39.Smith, H. K. & O'Kane, C. J. (1992) Roux's Arch. Dev. Biol. 200, 306–311. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, N., Zhang, J., Purcell, K. J., Cheng, Y. & Howard, K. (1997) Nature 385, 64–67. [DOI] [PubMed] [Google Scholar]
- 41.Park, M., Monsma, S. A. & Wolfner, M. F. (1994) Mech. Dev. 48, 51–57. [DOI] [PubMed] [Google Scholar]
- 42.DiBenedetto, A. J., Harada, H. A. & Wolfner, M. F. (1990) Dev. Biol. 139, 134–148. [DOI] [PubMed] [Google Scholar]
- 43.Monsma, S. A., Harada, H. A. & Wolfner, M. F. (1990) Dev. Biol. 142, 465–475. [DOI] [PubMed] [Google Scholar]
- 44.Trevitt, S., Fowler, K. & Partridge, L. (1988) J. Insect Physiol. 34, 821–828. [Google Scholar]
- 45.Liu, H. & Kubli, E. (2003) Proc. Natl. Acad. Sci. USA 100, 9929–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouletreau-Merle, J., Fouillet, P. & Terrier, O. (1992) Evol. Ecol. 6, 223–242. [Google Scholar]
- 47.Chapman, T., Hutchings, J. & Partridge, L. (1993) Proc. R. Soc. London Ser. B 253, 211–217. [DOI] [PubMed] [Google Scholar]