Abstract
Mating elicits two major changes in the reproductive behavior of many insect females. The egg-laying rate increases and the readiness to accept males (receptivity) is reduced. These postmating responses last ≈1 week in Drosophila melanogaster. Males that do not transfer sperm but transfer seminal fluid during mating induce a short-term response of 1 day. The long-term response of 1 week requires the presence of sperm (sperm effect). Hence, sperm is essential for the long-term persistence of the postmating responses. Three seminal fluid peptides elicit postmating responses: ovulin, sex-peptide (SP), and DUP99B. Using the technique of targeted mutagenesis by homologous recombination, we have produced males with mutant SP genes. Here, we report that males lacking functional SP elicit only a weak short-term response. However, these males do transfer sperm. Thus, (i) SP is the major agent eliciting the short-term and the long-term postmating responses and (ii) sperm is merely the carrier for SP. The second conclusion is supported by the finding that SP binds to sperm. The 36-aa-encoding SP gene is the first small Drosophila gene knocked out with the method of homologous recombination.
In many insect species, the reproductive behavior of females drastically changes after mating (1, 2). Egg laying is increased and receptivity is reduced. These female postmating responses are elicited by seminal fluid and sperm transferred during copulation. In most species, the virgin state is achieved again after some time (2). The egg-laying rate decreases to a virgin level and the female accepts courting males again. The coordination of egg production and egg laying with the presence of sperm in the female genital tract is largely in the interest of both sexes. However, it may be advantageous for a female to mate with more than one male to enhance the genetic diversity of her offspring. Female promiscuity can reduce the partner's fitness through sperm competition. As countermeasures, males have evolved mechanisms that reduce the females' tendency to remate (2–4). In some species, this may even lead to a reduced female lifespan (5). Experimental approaches have yielded insights into the nature of these sexual conflicts (6–11), but the molecular mechanisms involved remain largely unknown.
In Drosophila melanogaster, two male peptides induce the postmating responses when injected into virgin females: sexpeptide (SP, ACP70A) and ductus ejaculatorius peptide 99B (DUP99B; 99B stands for the cytological localization of the gene at position 99B; refs. 12 and 13). They are synthesized in the accessory glands and the ductus ejaculatorius, respectively. Both are transferred into the female during mating (3, 13). After entering the female genital tract, the two peptides are believed to be transferred into the hemolymph where they reach their targets (3, 13–15). This view is supported by ectopic, constitutive expression of SP in the fat body of females, leading to a continuous induction of the postmating responses (16). Although both peptides elicit both responses when injected, in vivo DUP99B seems to be of minor importance, as we did not find any differences between the postmating responses of females mated with WT males and males lacking the Dup99B gene, respectively (unpublished results). Ovulin (ACP26Aa) is another protein involved in the female postmating responses (17, 18). This protein stimulates increase of egg laying during the first day after mating, but it does not affect receptivity.
After a normal mating, the postmating responses of a D. melanogaster female last ≈1 week. Manning (19, 20) has shown that sperm is required for the persistence of both responses (sperm effect). Lack of sperm transfer results in a short-term response of 1 day as also observed after injection of physiological amounts of SP or DUP99B (12, 13). Therefore, a long-term response may be dependent (i) only on the presence of sperm or (ii) on a combination of sperm plus SP. Using the recently developed technique of targeted mutagenesis by homologous recombination (21–23), we have produced transgenic males lacking SP. We show that SP is the major agent eliciting the short-term and the long-term responses. DUP99B and ovulin play only minor roles on the first day after copulation. We conclude that sperm is merely the carrier for SP and that SP is the molecular basis of the sperm effect.
Materials and Methods
Original Vectors. The PCR cloning vector pGEM-T Easy was bought from Promega. The cloning vector pBS(Not) (22) and the targeting vector pTV2 (23) were both kindly supplied by K. G. Golic (University of Utah, Salt Lake City).
Donor Construct. Genomic DNA isolated from a y w stock of D. melanogaster was used as a PCR template. The flanking sequences of the SP gene were amplified as two PCR fragments. A fragment of ≈5.6 kb was amplified with an SpeI forward primer (5′-TTGATTTACTAGTCCTCATCCCATCTCGCTC-3′; SpeI recognition site underlined) and an EcoRI–AvrII reverse primer (5′-GGAGAATTCCTAGGCGAGTACGCAAACGAG-3′; EcoRI recognition site underlined; AvrII recognition site in italic; introduced point mutations in bold; Fig. 1A). Two point mutations were introduced into the reverse primer to produce a stop codon and an AvrII cutting site (Fig. 1E). A fragment of ≈6.1 kb was amplified with the AvrII primer (5′-TCGCCTAGGTCCAGTCCTGGGAATGGCCGT-3′; AvrII recognition site underlined; introduced point mutations in bold) and an XhoI reverse primer (5′-TGTCCTCGAGCAACAGACGGAGTTGCTTCAC-3′; XhoI recognition site underlined). Two point mutations were also introduced into the AvrII forward primer to produce a stop codon and an AvrII cutting site. These two fragments were cloned separately into the pGEMT easy vector. The first fragment was then ligated into the SpeI and EcoRI cutting sites of pBS(Not). This vector was dubbed pBS(Not)SP1. One I-SceI cutting site was added by annealing and inserting two complementary oligonucleotides (5′-CATGTAGGGATAACAGGGTAAT-3′ and 5′-CATGATTACCCTGTTATCCCTA-3′) into the NcoI cutting site of this fragment. This vector was dubbed pBS(Not)SP1/I-SceI. The second PCR fragment (see above) was then ligated to the AvrII and XhoI cutting sites of pBS(Not)SP1/I-SceI. This vector was dubbed pBS(Not)SP/I-SceI. Finally, we cloned the fragment into the NotI cutting site of pTV2 to obtain the targeting construct pTV2SP (Fig. 1 A).
Fig. 1.
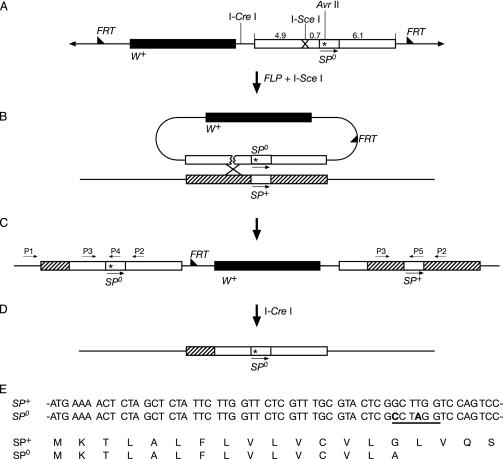
(A–D) Strategy for targeted knockout of SP gene (not drawn to scale). Only the essential steps are shown. Arrowheads show ends of P elements. Half-arrowheads show FRT sequence. Star indicates site of the introduced stop codon. Translation stops in the signal peptide (see below, E). (A) Targeting construct. Because SP is a small gene of ≈200 bp, we used the flanking sequences as donors (ends-in technique). Fragment length is in kilobases. (B) On activation with FLP recombinase, the targeting construct jumps out as a circle. It is then cut at the I-SceI cutting site and binds to the homologous sequence on a host chromosome. (C) Targeting at the SP+ gene produces tandem repeats containing an SP+ and an SP0 gene. P1–P5, primers. (D) By homologous recombination, the SP+ gene is deleted to obtain an SP0 null mutant. (E) Introduced mutations. SP+ and SP0, partial DNA sequences of the WT and the mutant SP genes, respectively. The sequence starts 5′ with the AUG encoding the first amino acid of the SP signal sequence. Changed bases in the mutant are bold. The newly introduced AvrII site is underlined. SP+ and SP0, amino acid sequences of SP translated from the WT and the mutant SP genes, respectively.
Fly Stocks. The stocks y w; 70FLP, 70I-SceI, Sco/Cyo and y w; 70I-CreI/TM3 were donated by Y. S. Rong (National Cancer Institute, Bethesda) and K. G. Golic. y w eyFLP was obtained from E. Hafen (University of Zurich, Zurich). Delta130/TM3, Sb, ry was donated by H. Bellen (Baylor College of Medicine, Houston). We used a VC (virginizer cross) virginizer line to collect virgin females (24). VC females were obtained by crossing females of the genotype y cm SxlM1; vir fl1 bw/SM5 with Oregon R WT males. This cross generates only VC females, i.e., virgin females, in great quantities.
Targeting Crosses. We used the donor inserted on the first and second chromosome. As described (21), the donor lines were crossed with y w; 70FLP, 70I-SceI, Sco/Cyo. The embryos were heat-induced during the early development stage. They were then crossed with eyFLP flies and screened for nonmosaic w+ flies. w+ flies contain homologous or nonhomologous integrations, as the ey-FLP completely removes any unexcised donor.
Verification of Targeting. PCR was used to verify the targeting. Primers P1 (5′-ATACGTTGCTGCCATCTGTCTC-3′; Fig. 1 C) and P2 (5′-GCGCTGCTAATTGCAAACG-3′) were used. P1 is outside of the homologous targeting sequence. A fragment of the same size can be amplified from the WT and the targeted allele. However, the enzyme AvrII cuts the fragment amplified from the targeted allele into two fragments. No fragment can be amplified from the nonhomologous targeted allele.
Reduction to a Single-Copy Mutant SP Gene. Flies with the targeted allele were crossed with 70I-CreI/TM3 flies. The progeny were heat-shocked when 2–3 days old. Non-TM3 males were selected and crossed with 70I-CreI/TM3 flies. The non-TM3 white-eye progeny are likely to have the targeted allele reduced to one copy. Finally, the null mutant was screened by allele-specific PCR. The primers P3 (5′-GTCCCTTAGTCACATAGC-3′) and P5 (5′-TTCCCAGGACTGGACCAAGC-3′) amplify a 524-bp fragment from the WT allele. The primers P3 (5′-GTCCCTTAGTCACATAGC-3′) and P4 (5′-TTCCCAGGACTGGACCTAGG-3′) amplify a fragment of the same size from the mutant allele.
Verification of the Null Mutant. Western blots were used to verify the absence of the SP in homozygous SP0 mutants. Male genital tracts were dissected, and polyclonal SP or DUP99B antibodies were used to assay for SP or DUP99B, respectively. The ovulin antibody was obtained from M. Wolfner (Cornell University, Ithaca, NY).
Oviposition and Receptivity Test. For measuring oviposition, 5-day-old virgin VC females were mated with 5-day-old males of various genotypes. Eggs laid by individual females were counted in a 24-h period. Between 17 and 22 females were included in each experiment. Each experiment was repeated three times. The receptivity test was performed as described (12). Three females were placed in a vial with seven naïve 5-day-old Oregon R males. Receptivity was calculated as the percentage of females that mated within 1 h. At least 24 females were observed per experiment. Every experiment was repeated twice.
Sperm Staining. Sperm receptacles and spermathecae were dissected as described (25). They were then stained in 2 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS.
Results
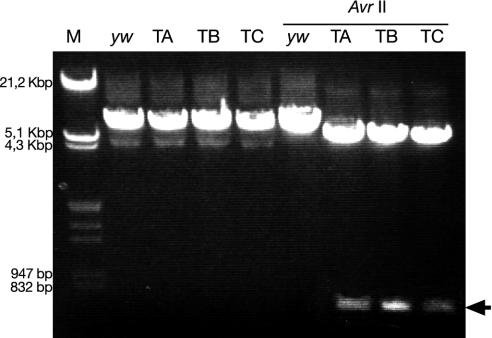
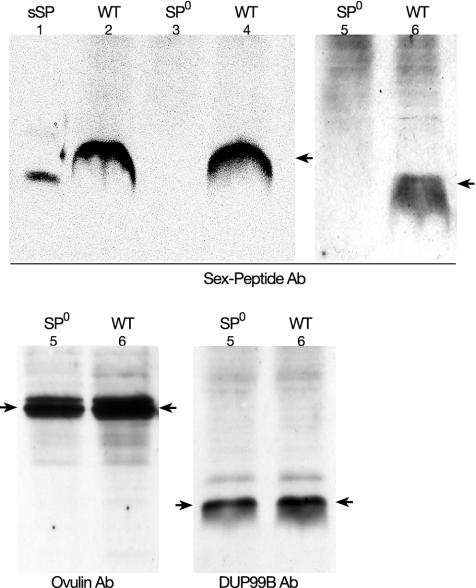
Generation of the Mutant Allele for SP. The WT D. melanogaster SP gene was replaced by a mutant gene by homologous targeting (Fig. 1 A–D). The frequency of homologous targeting is related to the length of the donor (23, 26, 27). Because SP is a small gene of ≈200 bp, we used the flanking sequences as donors (ends-in technique; refs. 21–23). We introduced two point mutations into the coding sequence of the SP signal peptide, resulting in one stop codon and one AvrII cutting site (Fig. 1E). Thus, in the SP0 mutant the SP gene is transcribed, but translation stops in the ORF of the signal sequence of the precursor. As a consequence, no mature SP is made. The targeting plasmid pTV2SP was injected into y w flies (Fig. 1 A). Two transgenic lines were recovered, one on the X, and another on the second chromosome. Because of the higher efficiency of targeting in the female germ line, most of the targeting was done in females. For the donor on the X chromosome, we screened 300 tubes (≈200 progeny per tube) and did not find any targeting. For the donor on the second chromosome, we screened 400 tubes and obtained three targets. We also tried to target the SP gene in male germ lines. In this latter screen, we used the donor on the second chromosome. No targeting was found in 300 tubes. Correct targeting was tested by PCR. Fig. 2 shows that all fragments derived from the three lines were cut by AvrII. Thus, the targeting was precise. This precision was verified by sequencing the amplified fragments. During the targeting procedure the SP gene is duplicated (Fig. 1 B and C). One copy contains the introduced point mutations, and the other is WT. To obtain an SP0 mutant, the WT copy has to be deleted. This end was achieved by classical homologous recombination induced by the I-CreI enzyme (Fig. 1D). Induction of the deletion was very efficient. White-eyed flies were found in most tubes, and about half of them had only the mutant SP copy. Western blots showed that SP is not present in SP0 males (Fig. 3). However, SP is present in the line SP0, SP+. These males contain copies of WT and mutant SP genes on the same chromosome (Fig. 1B). This line has the same genetic background as the SP0 line and hence is a good control.
Fig. 2.
Verification of homologous targeting. PCR was done with the primers P1 and P2 as indicated in Fig. 1C. M, λ DNA digested with EcoRI and HindIII. Lanes yw, TA, TB, and TC show the fragments amplified from DNA of the yw stock and the targeted lines TA, TB, and TC, respectively. Lanes overlined with AvrII, amplified DNA of the before-mentioned lines cut with AvrII. The AvrII site was introduced at the mutagenesis step. The arrow indicates a 729-bp fragment specific for targeted lines after cutting the DNA with AvrII enzyme.
Fig. 3.
Western blots showing the expression patterns of SP, ovulin (ACP26Aa), and DUP99B in different stocks. The nature of the polyclonal antibody used as probes is indicated. The expected phenotype for SP (WT or SP0) is indicated at the top of the corresponding lanes. Ovulin and DUP99B are expressed independent of the presence or absence of the SP gene. The numbers indicate the nature or source of the samples: 1, synthetic SP (sSP); 2–6, genotypes of the accessory gland extracts; 2, SP+/SP+; 3, SP0/SP0; 4, SP0, SP+/SPΔ; 5, SP+/SPΔ; and 6, SP0, SP+/SP0. Arrows indicate SP, ovulin, and DUP99B, respectively.
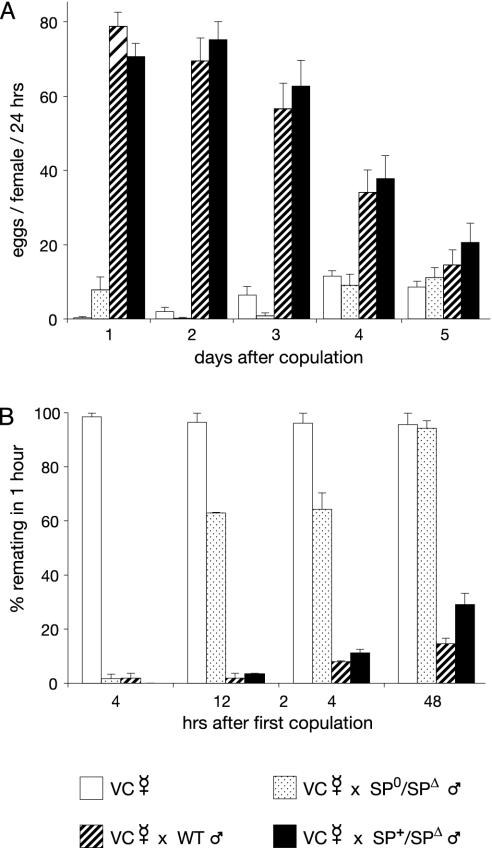
Postmating Responses of Females Mated to Males Lacking SP. Males containing the deficiency Δ130 (uncovering the SP gene, SPΔ) on one chromosome and the SP0 allele on the homologous chromosome were used to determine the contribution of SP to the postmating responses (SPΔ/SP0 males, hereafter dubbed SP null males). Homozygous SP0/SP0 males are homosexual. This homosexuality is very likely due to an unidentified recessive gene residing on the targeted chromosome. Virgin females deposit only a few eggs every day (Fig. 4A). Oviposition by females mated with Oregon R WT males or with heterozygous control males increased considerably in the first 4 days. Females mated with the SP null males, however, showed only a slight increase in oviposition on day 1. On the following days, the oviposition rate does not differ from the rate of unmated females. Sexually mature virgin females readily mate (Fig. 4B). Females mated with the control males showed strong reduction of receptivity in the first 48 h (Fig. 4B). However, for the females mated with the SP null mutant, we observed a strong reduction of receptivity only 4 h after copulation. Assayed 12 and 24 h after copulation, receptivity was >60%. After 48 h, receptivity had again increased to virgin levels. These effects are not due to lack of sperm. Sperm of the SP null males are motile and are transferred and stored correctly. Because fewer eggs are laid, fewer sperm are used in the females mated with these males. Thus, a considerable amount of sperm is left in the female genital tract even 9 days after copulation (Fig. 5B). In some females, sperm is present for fertilization for at least 20 days after copulation (results not shown). Furthermore, the peptides DUP99B and ACP26Aa are expressed normally in the SP null males (Fig. 3). Hence, knocking out the SP gene does not affect the expression of the two other peptides known to elicit the postmating responses.
Fig. 4.
Effects of SP on oviposition and receptivity. In contrast to females mated to WT males, females mated to males lacking SP drastically reduce oviposition and regain receptivity quickly. (A) Time course of average number of eggs laid per day by virgin females and females mated once to Oregon R WT, SP0/SPΔ, and SP0, SP+/SPΔ males, respectively. (B) Time course of receptivity of virgin females and females mated once to Oregon R WT males, SP0/SPΔ, and SP0, SP+/SPΔ males, respectively. SP0,SP+/SPΔ males produce the same amount of SP as Oregon R WT males. SP0/SPΔ males do not synthesize functional SP. All females were collected from a virginizer stock (VC). Standard errors are indicated.
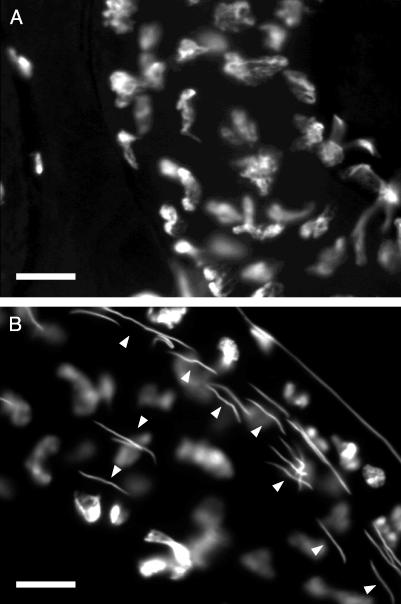
Fig. 5.
Sperm is transferred by males lacking functional SP and stored for many days. Sections through female seminal receptacles 9 days after matings with an SP0, SP+/SPΔ control male (A) and an SP0/SPΔ male lacking SP (B), respectively. Whereas no sperm can be found in the section of the female mated with the control male, many sperm can be identified in the female mated with the male lacking SP. The DNA of the sperm heads was stained with 4′,6-diamidino-2-phenylindole. (Magnification: ×1,000; bar = 10 μm.) Arrowheads point to sperm nuclei. Females were collected from a virginizer stock (VC).
Discussion
After copulation without sperm transfer, the short-term responses are fully induced, but only for 1 day (19). Without SP transfer, oviposition is barely induced, and only on the first day (Fig. 4A). Because the expressions of ovulin and DUP99B are not affected in the SP null males (Fig. 3 C and D), these two peptides may be responsible for the weak increase in oviposition observed on the first day after a mating with SP null males. The increase in egg laying observed in females mated to these males after day 3 corresponds to the increase in egg laying also observed over the same time period in virgin females (Fig. 4A). However, in contrast to the eggs laid by virgin females, these eggs are fertilized and produce offspring (results not shown). Indeed, offspring are obtained from eggs laid many days after copulation, demonstrating that the stored sperm of the SP null males is viable and functional (Fig. 5). Receptivity is strongly reduced only in the first few hours after mating (Fig. 4B). The short reduction of receptivity in matings with SP null males is very likely induced by DUP99B, as ovulin affects only oviposition. Because the postmating responses observed after copulation with SP null males are only weakly induced, we believe that they are not elicited by any other, unidentified, peptides or proteins. Thus, DUP99B and ovulin act only on the first day after mating and have a weak effect in comparison with SP; i.e., SP is the crucial peptide eliciting the short-term response. Because after day 1 only SP is active, and sperm alone cannot elicit the responses, SP is also the molecular agent of the sperm effect. Below, we propose a mechanism for the interaction between SP and sperm.
Our results are similar to those obtained by Chapman et al. (28), who used RNA interference to knock down SP levels. Females mated to SP knock-down males produced by RNA interference were significantly more receptive at 24 and 28 h after mating than females mated to control males. By 48 h, receptivity in mates of SP knock-down males was similar to that of virgin females. The rate of egg laying in females mated to SP knock-down males was significantly lower for 1–2 days after mating than for mates of control males, and then became indistinguishable from that of virgin females. The results of the RNA interference experiments showed a slightly longer initial stimulation of egg laying in mates of SP-deficient males than was found in this study. One factor contributing to these differences could be that the background rate of egg laying in virgin females was very different between the two studies. Thus, although there are slight differences in the magnitude of female postmating responses, the results of these two studies are qualitatively very similar.
Some years ago, we proposed a hypothesis about the molecular mechanism of the sperm effect (3). We suggested that sperm binds SP and, upon arrival in the female genital tract, releases SP continuously. Released SP then enters the hemolymph and reaches its targets (14, 15). Once sperm are used up, SP disappears too, and the female regains the virgin status. The hypothesis assumes that sperm acts as a carrier and that SP is the active molecule eliciting the two postmating responses. Here, we show that SP is indeed the molecular agent of the sperm effect, i.e., responsible for eliciting the two postmating responses. Using immunohistochemistry, Büsser (29) has shown that SP binds to sperm with its N-terminal region. Immediately after mating, SP binds to the head and tail of sperm. However, ≈5 days after copulation, SP bound to the tail is barely detected with a polyclonal antibody specific for the middle part of SP (J. Peng and E.K., unpublished results). Hence, it is very likely released from the sperm tail and subsequently enters the hemolymph to elicit the two postmating responses.
Reduction of the receptivity of a mated female by male compounds transferred during copulation is one way to avoid sperm competition. It has been proposed that the evolution of sperm length is a coevolved response to selection on the female reproductive tract (8). If the amount of SP transferred is proportional to the length of the sperm tail, long tails may have been evolutionarily favored because they carry more SP. We suggest SP binding to the sperm tail as an explanation for the excessive length of sperm tails in some Drosophila species. For example, the 3-mm-long male of Drosophila bifurca bears a sperm tail of 58 mm (30). The same reasoning may apply to other sperm-bound male substances (1, 31) that affect female reproductive traits in such a way that they enhance male reproductive success.
Acknowledgments
We thank D. Egli for many discussions; Y. Choffat for technical help and advice; S. Applebaum, G. Ribi, and D. Hosken for comments on the manuscript; E. Hafen, H. Bellen, and K. C. Golic for fly stocks and vectors; M. Wolfner for the ACP26Aa antibody; and J. Bangham and T. Chapman for comments on the manuscript and communication of unpublished results. This work was supported by Swiss National Science Foundation Grants 31 52440.97 and 31 64118.00 and grants from the Hescheler-Stiftung, the Julius Klaus-Stiftung, and the Stiftung für Wissenschaftliche Forschung der Universität Zürich (to E.K.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SP, sex-peptide; VC, virginizer cross.
See commentary on page 9643.
References
- 1.Chapman, T. (2001) Heredity 87, 511–521. [DOI] [PubMed] [Google Scholar]
- 2.Gillott, C. (2003) Annu. Rev. Entomol. 48, 163–184. [DOI] [PubMed] [Google Scholar]
- 3.Kubli, E. (1996) Adv. Dev. Biochem. 4, 99–128. [Google Scholar]
- 4.Wolfner, M. (1997) Insect Biochem. Mol. Biol. 27, 179–192. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. (1995) Nature 373, 241–244. [DOI] [PubMed] [Google Scholar]
- 6.Holland, B. & Rice, W. (1999) Proc. Natl. Acad. Sci. USA 96, 5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosken, D., Garner, T. & Ward, P. (2001) Curr. Biol. 11, 489–493. [DOI] [PubMed] [Google Scholar]
- 8.Miller, G. T. & Pitnick, S. (2002) Science 298, 1230–1233. [DOI] [PubMed] [Google Scholar]
- 9.Pitnick, S., Brown, W. & Miller, G. (2001) Proc. R. Soc. London Ser. B 268, 1–7. [Google Scholar]
- 10.Pitnick, S., Miller, G., Reagan, J. & Holland, B. (2001) Proc. R. Soc. London Ser. B 268, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice, W. (1996) Nature 381, 232–234. [DOI] [PubMed] [Google Scholar]
- 12.Chen, P., Stumm-Zollinger, E., Aigaki, T., Balmer, J., Bienz, M. & Böhlen, P. (1988) Cell 54, 291–298. [DOI] [PubMed] [Google Scholar]
- 13.Saudan, P., Hauk, K., Soller, M., Choffat, Y., Ottiger, M., Spörri, M., Ding, Z., Hess, D., Gehrig, P. M., Klauser, S., et al. (2002) Eur. J. Biochem. 269, 989–997. [DOI] [PubMed] [Google Scholar]
- 14.Ding, Z., Haussmann, I., Ottiger, M. & Kubli, E. (2003) J. Neurobiol. 55, 372–384. [DOI] [PubMed] [Google Scholar]
- 15.Ottiger, M., Soller, M., Stocker, R. F. & Kubli, E. (2000) J. Neurobiol. 44, 57–71. [PubMed] [Google Scholar]
- 16.Aigaki, T., Fleischmann, I., Chen, P. S. & Kubli, E. (1991) Neuron 7, 557–563. [DOI] [PubMed] [Google Scholar]
- 17.Chapman, T., Herndon, L. A., Heifetz, Y., Partridge, L. & Wolfner, M. F. (2001) Proc. R. Soc. London Ser. B 268, 1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon, L. A. & Wolfner, M. F. (1995) Proc. Natl. Acad. Sci. USA 92, 10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning, A. (1962) Nature 194, 252–253. [Google Scholar]
- 20.Manning, A. (1967) Anim. Behav. 15, 239–250. [DOI] [PubMed] [Google Scholar]
- 21.Rong, Y. S. & Golic, K., G. (2000) Science 288, 2013–2018. [DOI] [PubMed] [Google Scholar]
- 22.Rong, Y. S. & Golic, K., G. (2001) Genetics 157, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong, Y. S., Titen, S. W., Xie, H. B., Golic, M. M., Bastiani, M., Bandyopadhyay, P., Olivera, B. M., Brodsky, M., Rubin, G. M. & Golic, K. G. (2002) Genes Dev. 16, 1568–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilfiker, A., Amrein, H., Dubendorfer, A., Schneiter, R. & Nothiger, R. (1995) Development (Cambridge, U.K.) 121, 4017–4026. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert, D. G. (1981) Drosophila Inf. Serv. 56, 46–47. [Google Scholar]
- 26.Deng, C. & Capecchi, M. R. (1992) Mol. Cell. Biol. 12, 3365–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gloor, G. B. (2001) Trends Genet. 17, 549–551. [DOI] [PubMed] [Google Scholar]
- 28.Chapman, T., Bangham, J., Vinti, G., Seifried, B., Lung, O., Wolfner, M. F., Smith, H. K. & Partridge, L. (2003) Proc. Natl. Acad. Sci. USA 100, 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Büsser, S. (2000) Master's thesis (University of Zurich, Zurich).
- 30.Pitnick, S., Spicer, G. S. & Markow, T. A. (1995) Nature 375, 109 (lett.). [DOI] [PubMed] [Google Scholar]
- 31.Neubaum, D. M. & Wolfner, M. F. (1999) Genetics 153, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]