Abstract
The intermediate filament protein, nestin, marks progenitor cells of the CNS. Such CNS stem cells are selectively labeled by placing GFP under the control of the nestin regulatory sequences. During early anagen or growth phase of the hair follicle, nestin-expressing cells, marked by GFP fluorescence in nestin-GFP transgenic mice, appear in the permanent upper hair follicle immediately below the sebaceous glands in the follicle bulge. This is where stem cells for the hair follicle outer-root sheath are thought to be located. The relatively small, oval-shaped, nestin-expressing cells in the bulge area surround the hair shaft and are interconnected by short dendrites. The precise locations of the nestin-expressing cells in the hair follicle vary with the hair cycle. During telogen or resting phase and in early anagen, the GFP-positive cells are mainly in the bulge area. However, in mid- and late anagen, the GFP-expressing cells are located in the upper outer-root sheath as well as in the bulge area but not in the hair matrix bulb. These observations show that the nestin-expressing cells form the outer-root sheath. Results of the immunohistochemical staining showed that nestin, GFP, keratin 5/8, and keratin 15 colocalize in the hair follicle bulge cells, outer-root sheath cells, and basal cells of the sebaceous glands. These data indicate that nestin-expressing cells, marked by GFP, in the hair follicle bulge are indeed progenitors of the follicle outer-root sheath. The expression of the unique protein, nestin, in both neural stem cells and hair follicle stem cells suggests their possible relation.
Keywords: stem cells, hair cycle, neurological, GFP, imaging
Hair growth is a unique cyclic regeneration phenomenon. The hair follicle undergoes repeated cycles of periods of growth (anagen), regression (catagen), and rest (telogen) throughout the life of mammals. The progenitor or stem cells for the outer-root sheath of the hair follicle were thought to reside in the permanent upper portion of the hair follicle, the so-called bulge area (1, 2).
Recently Taylor et al. (3) reported that hair follicle bulge stem cells are potentially bipotent because they can give rise to not only cells of the hair follicle but also to epidermal cells. Other experiments (1) also have provided new evidence that the upper outer-root sheath of vibrissal (whisker) follicles of adult mice contains multipotent stem cells, which can differentiate into hair follicle matrix cells, sebaceous gland basal cells, and epidermis. Recently, Toma et al. (4) reported that multipotent adult stem cells isolated from mammalian skin dermis, termed skin-derived precursors, can proliferate and differentiate in culture to produce neurons, glia, smooth muscle cells, and adipocytes. However, the exact location of these stem cells in skin is unknown, and their functions are still unclear.
We report here the expression of nestin, a marker for neural progenitor cells, in the cells of the follicle bulge. Nestin was linked to GFP, allowing us to observe that the nestin-containing cells formed the major part of the hair follicle each cycle. This expression of the neural stem cell protein nestin in hair follicle stem cells suggests a possible relation.
Materials and Methods
Nestin-GFP Transgenic Mice. Nestin is an intermediate filament (IF) gene that is a marker for CNS progenitor cells and neuroepithelial stem cells (5). Enhanced GFP (EGFP) transgenic mice carrying EGFP under the control of the nestin second-intron enhancer are used for studying and visualizing the self-renewal and multipotency of CNS stem cells (5–7). Here we report that hair follicle stem cells strongly express nestin as evidenced by nestin-regulated EGFP expression.
Induction of Anagen. Nestin-regulated GFP transgenic mice, 6–8 weeks old, in the telogen phase of hair growth were depilated by a hot mixture of rosin and beeswax. Samples (5 × 5 mm2) were excised from the dorsal skin right before depilation (telogen) and at days 1–5 (early anagen), days 8 and 10 (middle anagen), days 14 and 15 (late anagen), and days 19 and 20 (catagen) after depilation. The skin samples were divided into two parts, one for fluorescence microscopy and the other for frozen sections. Briefly, the skin samples were embedded in tissue-freezing embedding medium and frozen at –80°C overnight. Sections 8 μm thick were cut with a Leica CM1850 cryostat. The frozen sections were air-dried and counter-stained with propidium iodide for fluorescence microscopy.
Fluorescence and Confocal Microscopy. The nestin-GFP skin samples, after dissecting out the s.c. tissue, were directly observed with dermis up and epidermis down under a Nikon fluorescent microscope equipped with GFP optics. An MRC-600 confocal imaging system (Bio-Rad) mounted on a Nikon Optiphot with a ×10 PlanApo objective was used also.
Immunohistochemical Staining. Colocalization of nestin, keratins 5, 8, and 15, and GFP in the paraffin-embedded C57Bl6 mouse and nestin-GFP transgenic mouse skin sections was detected with the DAKO ARK animal research kit (nestin and keratins) and DAKO EnVision doublestain system following manufacturer instructions. Briefly, the activity of endogenous peroxidase in the skin samples was quenched with incubation in a peroxidase-blocking solution for 5 min. The slide then was incubated with the prepared biotinylated primary antibody (GFP mAb, 1:100; nestin mAb, 1: 80; keratin 5/8 mAb, 1:250; and keratin 15 mAb, 1:100) for 15 min followed by incubation with streptavidin peroxidase for 15 min. The staining was completed by incubation with substrate-chromogen 3,3′-diaminobenzidine (DAB) or nuclear fast red for 5 min. Brown (DAB) or cherry-red (nuclear fast red) staining was used for antigen staining. Nestin mAb (rat 401) was purchased from the University of Iowa (Iowa City). Keratin 5/8 mAb (MAB3228) and keratin 15 mAb (CBL 272) were purchased from Chemicon.
Results and Discussion
The cells with nestin-controlled GFP expression are located in the permanent upper region of telogen hair follicles immediately below the sebaceous glands and in the bulge area (Figs. 1, 2, 3). These cells are relatively small, oval- or round-shaped, and interconnected by dendrite-like structures (Fig. 2).
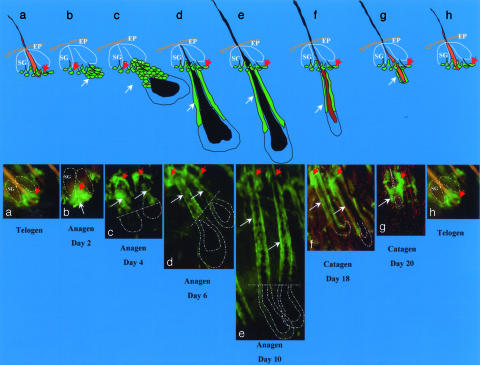
Fig. 1.
Hair follicle stem cells in the hair-growth cycle. (Upper) Cartoon showing position of nestin-GFP stem cells at each stage of the hair follicle cycle. SG, sebaceous gland; EP, epidermis. (Lower a) Nestin-GFP-expressing hair follicle stem cells (red arrow) located in the hair follicle bulge area in telogen phase. (b) Day 2 after anagen induction by depilation. Note the new hair follicle cells (white arrow) formed directly from the bulge nestin-GFP-expressing stem cells. (c–e)Day4(c), day 6 (d), and day 10 (e) after anagen induction by depilation. Note the nestin-GFP-expressing outer-root sheath cells (white arrows) in the upper two-thirds of the hair follicle. (f and g) Day 19 (f) and day 20 (g) after depilation. Note in f and g that the hair follicles are in the catagen phase and are undergoing regression and degeneration, including the nestin-GFP-expressing cells in the outer-root sheath. The bulge area nestin-GFP-expressing stem cells remain. (h) Hair follicle cycling in telogen phase.
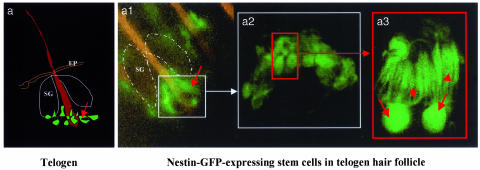
Fig. 2.
Hair follicle nestin-GFP-expressing cells in the telogen phase of nestin-GFP transgenic mouse skin. The skin sample was prepared freshly right after excision from the back skin of a nestin-GFP transgenic mouse. The skin sample then was directly observed by fluorescence or confocal microscopy with the dermis side up after s.c. tissue was dissected out. (a) Cartoon of telogen hair follicle showing position of nestin-GFP-expressing hair follicle stem cells. (a1) Low-magnification fluorescence microscopy image showing the ring of bulge nestin-GFP-expressing stem cells (small white box, see a). (a2) High-magnification confocal microscopy image reflecting the small white box in a1. Note the small round- or oval-shaped nestin-GFP-expressing cells in the bulge area of the hair follicle (small red box). (a3) High-magnification fluorescence microscopy image showing two individual nestin-GFP-expressing stem cells reflecting the red box in a2. Note the unique morphology of the hair follicle stem cells and multiple dendrite-like structures of each cell. Red arrows indicate the cell body, and red arrowheads show the multiple dendritic structure of each cell. (Original magnifications: a1, ×100; a2, ×400; a3, ×1,600.) SG, sebaceous gland; EP, epidermis.
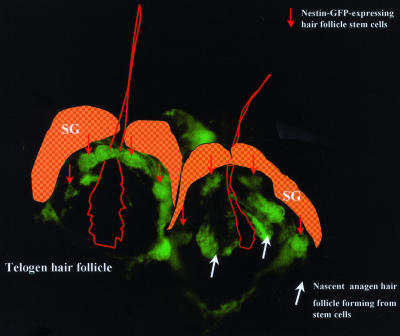
Fig. 3.
Telogen-anagen transition showing hair follicle stem cells forming nascent hair follicle. The image was taken by fluorescence microscopy 18 h after depilation. Note the bulge nestin-GFP-expressing hair follicle stem cells in the telogen phase (left hair follicle, red arrows) and the nascent anagen hair follicle directly formed from the bulge nestin-GFP-expressing stem cells (right hair follicle, white arrows). (Original magnification, ×400.)
The location and number of the nestin-expressing cells is hair-cycle-dependent (Fig. 1). The progression and proliferation of the GFP-marked, nestin-producing cells in the developing hair follicle was followed in detail in mice (6–8 weeks old) after inducing anagen in telogen follicles by depilation. As previously described for hair follicle stem cells (1–3), at telogen the green fluorescent, nestin-expressing cells in the hair follicle are located only at the upper permanent bulge region (Figs. 1a, 2, and 3). Two to 3 days after depilation, nestin-expressing hair follicle cells have proliferated, migrating down from the bulge (Figs. 1 b and c and 3). During the middle and late anagen phases, the nestin-expressing hair follicle cells occupy the upper two-thirds of the outer-root sheath and are absent from the lower one-third of the follicle and the hair matrix bulb (Fig. 1 c–e). Fig. 1 c–g shows nestin-expressing outer-root sheath cells during the entire anagen and catagen phases. In catagen, when hair bulb matrix cells undergo regression and degeneration, the number of outer-root sheath nestin-GFP-expressing cells decreases along with shrinkage of the hair follicle. Eventually, by the next telogen these cells localize only in the bulge (Fig. 1h).
The data indicate that the nestin-expressing cells include the true progenitor or stem cells of the hair follicle outer-root sheath. At the peak of anagen, fully two-thirds of the length of the follicle outer-root sheath is composed of nestin-expressing GFP-fluorescent cells. These apparently originate in the small cluster of nestin-expressing cells in the telogen follicle and proliferate with a kinetics synchronous with the hair cycle (Figs. 1 and 3). Most of the anagen follicle outer-root sheath must derive from these putative stem cells, because significant recruitment of cells from surrounding tissue seems unlikely in view of the physical, physiological, and temporal barriers. These results provide a depiction of living stem cells forming a critical part the new hair follicle structure (Figs. 1 b and c and 3).
Our results are strongly supported by the findings of others. Recently, Oshima et al. (1) reported that the upper region of the outer-root sheath of vibrissal follicles of adult mice contains multipotent stem cells that respond to morphogenic signals to generate multiple hair follicles, sebaceous glands, and epidermis. These findings agree with our observations of nestin-GFP expression in the outer-root sheath.
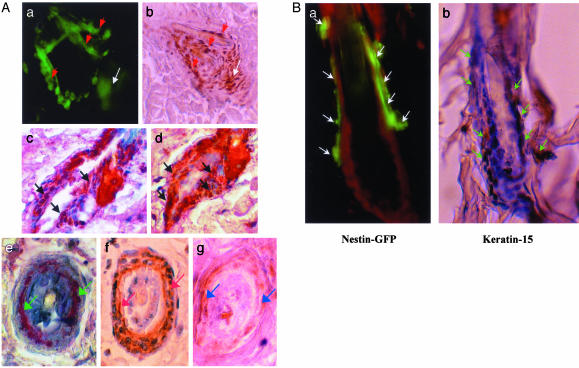
These nestin-GFP-expressing hair follicle progenitor or stem cells also express keratin 5/8 and keratin 15, which are potential markers of hair follicle stem cells (8). Results of immunohistochemical staining (Fig. 4) show that nestin, GFP, and keratins 5/8 and 15 colocalize in the hair follicle bulge cells, outer-root sheath cells, and basal cells of the sebaceous glands. These data further support the role of nestin-GFP-expressing cells in the hair follicle bulge as the progenitors of the outer-root sheath.
Fig. 4.
(A) Colocalization of GFP, nestin, and keratins 5/8 and 15 in hair follicle bulge stem cells and outer-root sheath cells determined by immunohistochemical staining. (a) Confocal image of live tissue showing nestin-GFP-expressing hair follicle bulge stem cells (red arrows) forming the nascent anagen hair follicle (white arrow). (b) Paraffin-embedded tissue section immunohistochemically stained with nestin antibody showing the colocalization of nestin expression in the nascent hair follicle and bulge stem cells. Red arrows indicate nestin-positive hair follicle bulge stem cells. The white arrow indicates nestin-positive nascent hair follicle. (c and d) Two serial paraffin-embedded longitudinal sections of nestin-GFP transgenic mouse hair follicles double-immunohistochemical-stained with GFP mAb and keratin 15 mAb. GFP is detected by chromogen fast red. Keratin 15 is detected by chromogen 3,3′-diaminobenzidine (brown). Note the colocalization of nestin-GFP and keratin 15 in the bulge hair follicle stem cells as seen by the reddish brown color (black arrows). (e–g) Serial paraffin-embedded cross sections of nestin-GFP transgenic mouse hair follicles immunohistochemically stained with GFP mAb (e, green arrows), keratin 5/8 mAb (f, white arrows), and nestin mAb (g, blue arrows). Note the colocalization of GFP, keratin 5/8, and nestin in the outer-root sheath cells of hair follicles by comparing e–g. (Magnification, ×400.) (B) Colocalization of nestin-GFP and keratin 15 in telogen hair follicle stem cells. (a) Fluorescence microscopy of a frozen section of bulge area of telogen hair follicle from the nestin-GFP transgenic mouse counterstained with propidium iodide. Note the nestin-GFP-expressing hair follicle stem cells (white arrows). (Magnification, ×400.) (b) Serial section of that shown in a immunohistochemical-stained with keratin 15 mAb. Note that the expression of keratin 15 (brown stain, green arrows) colocalizes with nestin-GFP in a. Slides were counterstained with hematoxylin and observed by light microscopy. (Magnification, ×400.)
The recent upsurge of interest in hair follicle biology has revealed a surprising complexity of functions and cell types in addition to the obvious role in forming the hair shaft (1–3). Here we report the observation that outer-root sheath progenitor cells in the follicle share the nestin marker previously found in neural stem cells. This finding hints at a possible relation between the hair follicle cells and neural stem cells. The data also prove what has previously been suspected, i.e., that the bulge cells that we have shown to express nestin-GFP proliferate to form much of the outer-root sheath during the anagen growth phase. Of course, it is possible that the nestin-expressing cells play a much wider role and serve as stem cells for the entire hair follicle. In this case, the remaining portions of the follicle, e.g., the inner root sheath and the matrix, would originate from the nestin-expressing cells but would lose nestin expression as differentiation proceeded.
References
- 1.Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233–245. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis, G., Sun, T.-T. & Lavker, R. M. (1990) Cell 61, 1329–1337. [DOI] [PubMed] [Google Scholar]
- 3.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T.-T. & Lavker, R. M. (2000) Cell 102, 451–461. [DOI] [PubMed] [Google Scholar]
- 4.Toma, J. G., Akhavan, M., Fernandes, J. L., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. & Miller, F. D. (2001) Nat. Cell Biol. 3, 778–784. [DOI] [PubMed] [Google Scholar]
- 5.Lendahl, U., Zimmerman, L. B. & McKay, R. D. G. (1990) Cell 60, 585–595. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman, L., Parr, B., Lendahl, U., Cunningham, M., McKay, R., Gavin, B., Mann, J., Vassileva, G. & McMahon, A. (1994) Neuron 12, 11–24. [DOI] [PubMed] [Google Scholar]
- 7.Yaworsky, P. J. & Kappen, C. (1999) Dev. Biol. 205, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyle, S., Christofidou-Solomidou, M., Liu, Y., Elder, D. E., Albelda, S. & Cotsarelis, G. (1999) J. Invest. Dermatol. Symp. Proc. 4, 296–301. [DOI] [PubMed] [Google Scholar]