Abstract
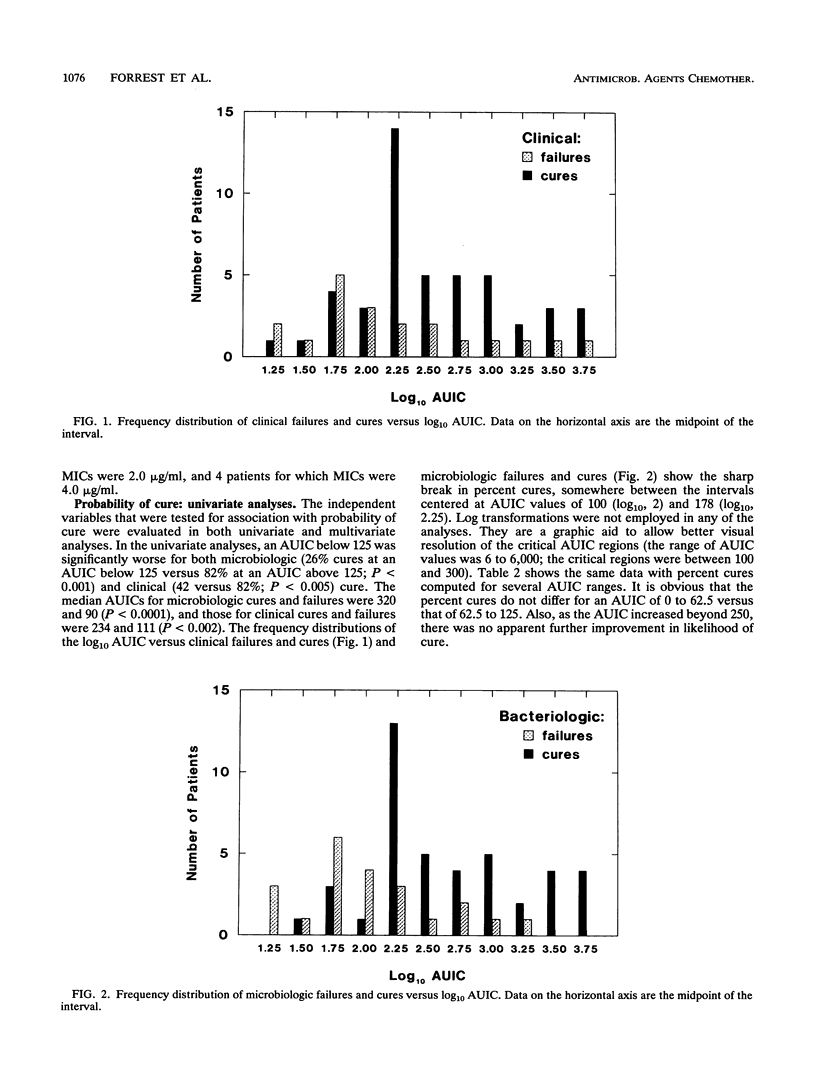
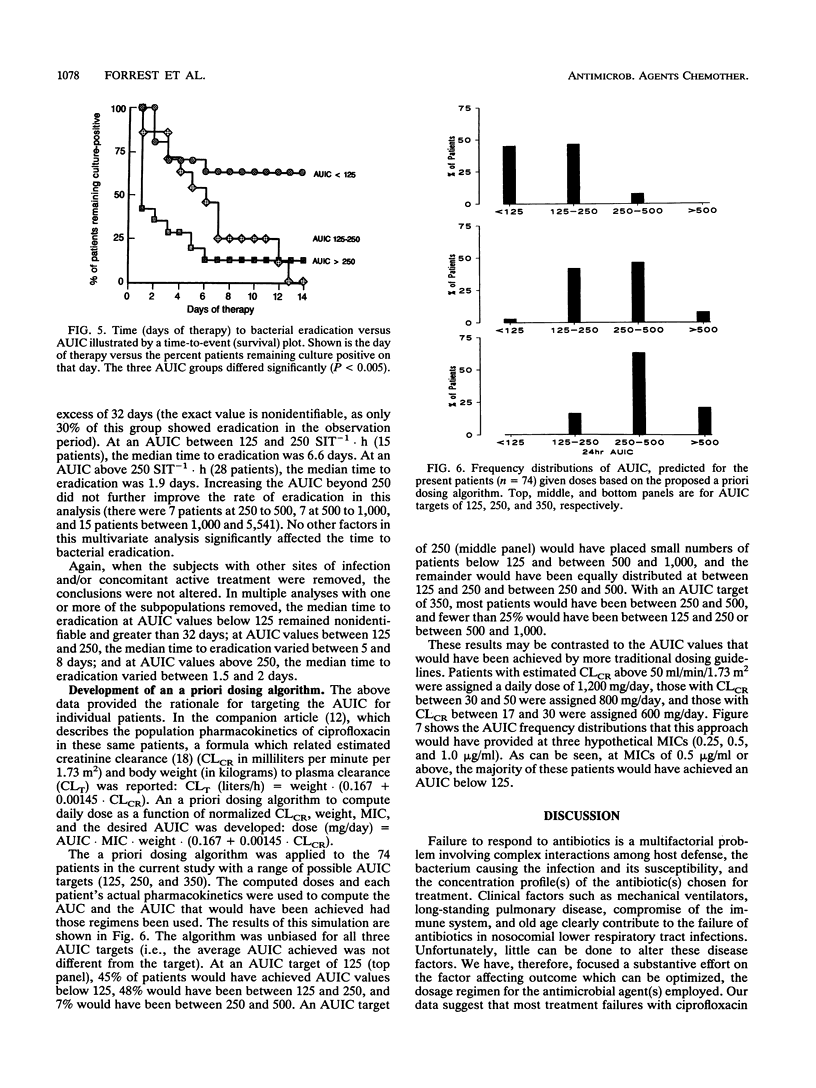
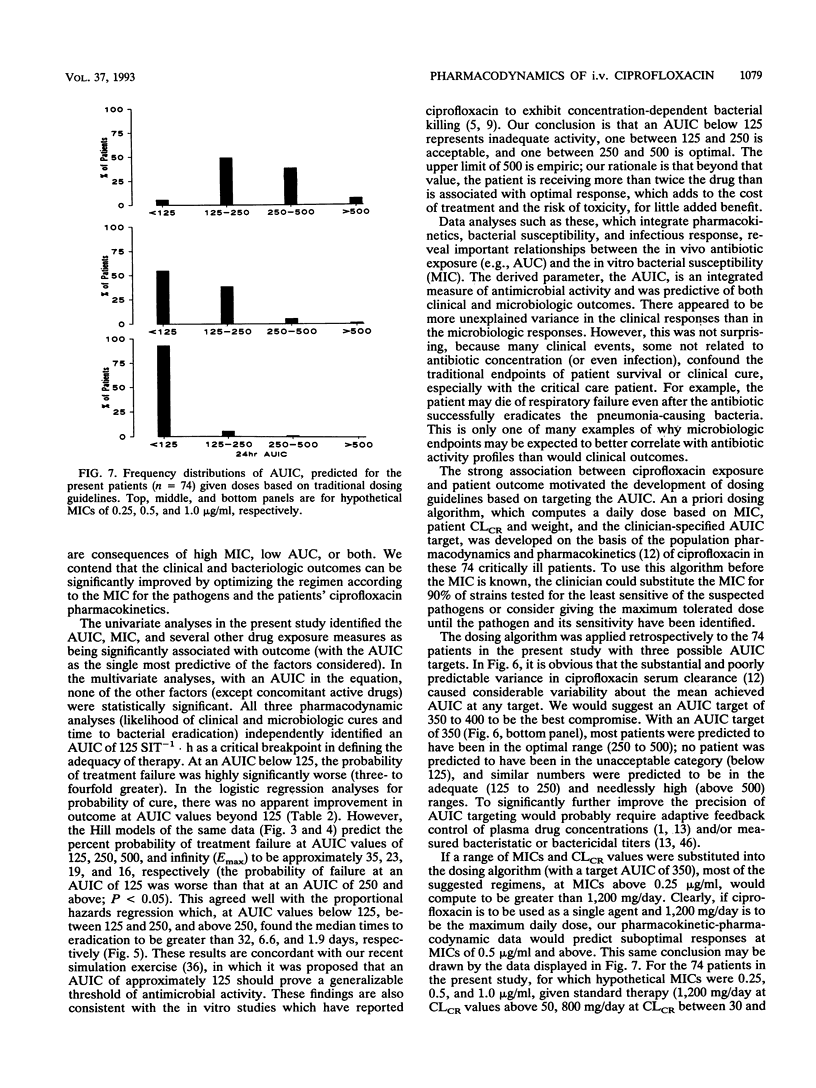
Seventy-four acutely ill patients were treated with intravenous ciprofloxacin at dosages ranging between 200 mg every 12 h and 400 mg every 8 h. A population pharmacokinetic-pharmacodynamic analysis relating drug exposure (and other factors) to infectious outcome was performed. Plasma samples were obtained and assayed for ciprofloxacin by high-performance liquid chromatography. Samples from patients were frequently cultured so that the day of bacterial eradication could be determined. The pharmacokinetic data were fitted by iterative two-stage analysis, assuming a linear two-compartment model. Logistic regression was used to model ciprofloxacin exposure (and other potential covariates) versus the probabilities of achieving clinical and microbiologic cures. The same variables were also modelled versus the time to bacterial eradication by proportional hazards regression. The independent variables considered were dose, site of infection, infecting organism and the MIC for it, percent time above the MIC, peak, peak/MIC ratio, trough, trough/MIC ratio, 24-h area under the concentration-time curve (AUC), AUC/MIC ratio (AUIC), presence of other active antibacterial agents, and patient characteristics. The most important predictor for all three measures of ciprofloxacin pharmacodynamics was the AUIC. A 24-h AUIC of 125 SIT-1.h (inverse serum inhibitory titer integrated over time) was found to be a significant breakpoint for probabilities of both clinical and microbiologic cures. At an AUIC below 125 (19 patients), the percent probabilities of clinical and microbiologic cures were 42 and 26%, respectively. At an AUIC above 125 (45 patients), the probabilities were 80% (P < 0.005) and 82% (P < 0.001), respectively. There were two significant breakpoints in the time-to-bacterial-eradication data. At an AUIC below 125 (21 patients), the median time to eradication exceeded 32 days; at an AUIC of 125 to 250 (15 patients), time to eradication was 6.6 days: and at AUIC above 250 (28 patients), the median time to eradication was 1.9 days (groups differed; P < 0.005). These findings, when combined with pharmacokinetic data reported in the companion article, provide the rationale and tools needed for targeting the dosage of intravenous ciprofloxacin to individual patients' pharmacokinetics and their bacterial pathogens' susceptibilities. An a priori dosing algorithm (based on MIC, patient creatine clearance and weight, and the clinician-specified AUIC target) was developed. This approach was shown, retrospectively, to be more precise than current guidelines, and it can be used to achieve more rapid bacteriologic and clinical responses to ciprofloxacin, as a consequence of targeting the AUIC.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger D. M., Peterson L. R., Gerding D. N., Moody J. A., Fasching C. E. Ciprofloxacin, azlocillin, ceftizoxime and amikacin alone and in combination against gram-negative bacilli in an infected chamber model. J Antimicrob Chemother. 1986 Jul;18(1):51–63. doi: 10.1093/jac/18.1.51. [DOI] [PubMed] [Google Scholar]
- Barriere S. L., Ely E., Kapusnik J. E., Gambertoglio J. G. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985 Jul;16(1):49–59. doi: 10.1093/jac/16.1.49. [DOI] [PubMed] [Google Scholar]
- Cars O. Pharmacokinetics of antibiotics in tissues and tissue fluids: a review. Scand J Infect Dis Suppl. 1990;74:23–33. [PubMed] [Google Scholar]
- Chalkley L. J., Koornhof H. J. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother. 1985 Aug;28(2):331–342. doi: 10.1128/aac.28.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G., Standiford H., Ryan P., McNamee W., Tatem B., Schimpff S. Correlation of predicted serum bactericidal activities and values measured in volunteers. Eur J Clin Microbiol. 1986 Feb;5(1):88–92. doi: 10.1007/BF02013475. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner P. D., Neu H. C. The inhibitory quotient. A method for interpreting minimum inhibitory concentration data. JAMA. 1981 Oct 2;246(14):1575–1578. doi: 10.1001/jama.246.14.1575. [DOI] [PubMed] [Google Scholar]
- Forrest A., Ballow C. H., Nix D. E., Birmingham M. C., Schentag J. J. Development of a population pharmacokinetic model and optimal sampling strategies for intravenous ciprofloxacin. Antimicrob Agents Chemother. 1993 May;37(5):1065–1072. doi: 10.1128/aac.37.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuursted K., Gerner-Smidt P. Analysis of the interaction between piperacillin and ciprofloxacin or tobramycin against thirteen strains of Pseudomonas aeruginosa, using killing curves. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):193–197. doi: 10.1111/j.1699-0463.1987.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Hyatt D. S., Rogers T. R., McCarthy D. M., Samson D. S. A randomized trial of ciprofloxacin plus azlocillin versus netilmicin plus azlocillin for the empirical treatment of fever in neutropenic patients. J Antimicrob Chemother. 1991 Aug;28(2):324–326. doi: 10.1093/jac/28.2.324. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Padula A. M., Kimbrough R. C., Jones S. R., Chaisson R. E., Mills J., Sande M. A. Infectious complications with respiratory pathogens despite ciprofloxacin therapy. N Engl J Med. 1991 Aug 15;325(7):520–521. doi: 10.1056/nejm199108153250719. [DOI] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Pechère J. C., Marchou B., Auckenthaler R. Combination therapy: a way to limit emergence of resistance? Am J Med. 1986 Jun 30;80(6B):138–142. doi: 10.1016/0002-9343(86)90491-2. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Bacterial resistance to fluoroquinolones. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S57–S63. doi: 10.1093/clinids/10.supplement_1.s57. [DOI] [PubMed] [Google Scholar]
- Nix D. E., De Vito J. M., Schentag J. J. Liquid-chromatographic determination of ciprofloxacin in serum and urine. Clin Chem. 1985 May;31(5):684–686. [PubMed] [Google Scholar]
- Nix D. E., Goodwin S. D., Peloquin C. A., Rotella D. L., Schentag J. J. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother. 1991 Oct;35(10):1953–1959. doi: 10.1128/aac.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D. E., Goodwin S. D., Peloquin C. A., Rotella D. L., Schentag J. J. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrob Agents Chemother. 1991 Oct;35(10):1947–1952. doi: 10.1128/aac.35.10.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D. E., Sands M. F., Peloquin C. A., Vari A. J., Cumbo T. J., Vance J. W., Fracasso J. E., Schentag J. J. Dual individualization of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Am J Med. 1987 Apr 27;82(4A):352–356. [PubMed] [Google Scholar]
- Nix D. E., Spivey J. M., Norman A., Schentag J. J. Dose-ranging pharmacokinetic study of ciprofloxacin after 200-, 300-, and 400-mg intravenous doses. Ann Pharmacother. 1992 Jan;26(1):8–10. doi: 10.1177/106002809202600101. [DOI] [PubMed] [Google Scholar]
- Peloquin C. A., Cumbo T. J., Nix D. E., Sands M. F., Schentag J. J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med. 1989 Oct;149(10):2269–2273. [PubMed] [Google Scholar]
- Righter J. Ciprofloxacin treatment of Staphylococcus aureus infections. J Antimicrob Chemother. 1987 Oct;20(4):595–597. doi: 10.1093/jac/20.4.595. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. Overview of preclinical studies with ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):2–11. [PubMed] [Google Scholar]
- Schentag J. J. Clinical significance of antibiotic tissue penetration. Clin Pharmacokinet. 1989;16 (Suppl 1):25–31. doi: 10.2165/00003088-198900161-00005. [DOI] [PubMed] [Google Scholar]
- Schentag J. J. Correlation of pharmacokinetic parameters to efficacy of antibiotics: relationships between serum concentrations, MIC values, and bacterial eradication in patients with gram-negative pneumonia. Scand J Infect Dis Suppl. 1990;74:218–234. [PubMed] [Google Scholar]
- Schentag J. J., Nix D. E., Adelman M. H. Mathematical examination of dual individualization principles (I): Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP. 1991 Oct;25(10):1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Smith I. L., Swanson D. J., DeAngelis C., Fracasso J. E., Vari A., Vance J. W. Role for dual individualization with cefmenoxime. Am J Med. 1984 Dec 21;77(6A):43–50. doi: 10.1016/s0002-9343(84)80074-1. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Neu H. C., Parry M. F., Mandell W. Oral ciprofloxacin therapy of infections due to Pseudomonas aeruginosa. Lancet. 1986 Apr 12;1(8485):819–822. doi: 10.1016/s0140-6736(86)90937-2. [DOI] [PubMed] [Google Scholar]
- Steimer J. L., Mallet A., Golmard J. L., Boisvieux J. F. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev. 1984;15(1-2):265–292. doi: 10.3109/03602538409015066. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Franke J. J., Weeks L. S., Manion F. A. Comparison of the bactericidal activity of ciprofloxacin alone and in combination with selected antipseudomonal beta-lactam agents against clinical isolates of Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1988 Sep;11(1):41–52. doi: 10.1016/0732-8893(88)90072-7. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Reller L. B. Correlation of serum bactericidal activity with antimicrobial agent level and minimal bactericidal concentration. J Infect Dis. 1982 Feb;145(2):160–168. doi: 10.1093/infdis/145.2.160. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Bacterial resistance to quinolones: mechanisms and clinical importance. Rev Infect Dis. 1989 Jul-Aug;11 (Suppl 5):S960–S968. doi: 10.1093/clinids/11.supplement_5.s960. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Swartz M. N. Drug therapy. Serum bactericidal activity as a monitor of antibiotic therapy. N Engl J Med. 1985 Apr 11;312(15):968–975. doi: 10.1056/NEJM198504113121507. [DOI] [PubMed] [Google Scholar]



