Abstract
Diminished apoptosis, a critical event in tumorigenesis, is linked to down-regulated 15-lipoxygenase-1 (15-LOX-1) expression in colorectal cancer cells. 13-S-hydroxyoctadecadienoic acid (13-S-HODE), which is the primary product of 15-LOX-1 metabolism of linoleic acid, restores apoptosis. Nonsteroidal antiinflammatory drugs (NSAIDs) transcriptionally up-regulate 15-LOX-1 expression to induce apoptosis. Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors for linoleic and arachidonic acid metabolites. PPAR-δ promotes colonic tumorigenesis. NSAIDs suppress PPAR-δ activity in colon cancer cells. The mechanistic relationship between 15-LOX-1 and PPAR-δ was previously unknown. Our current study shows that (i) 13-S-HODE binds to PPAR-δ, decreases PPAR-δ activation, and down-regulates PPAR-δ expression in colorectal cancer cells; (ii) the induction of 15-LOX-1 expression is a critical step in NSAID down-regulation of PPAR-δ and the resultant induction of apoptosis; and (iii) PPAR-δ is an important signaling receptor for 13-S-HODE-induced apoptosis. The in vivo relevance of these mechanistic findings was demonstrated in our tumorigenesis studies in nude mouse xenograft models. Our findings indicate that the down-regulation of PPAR-δ by 15-LOX-1 through 13-S-HODE is an apoptotic signaling pathway that is activated by NSAIDs.
Apoptosis, an important regulatory event that maintains homeostasis in normal epithelial cells (1), is diminished in human cancers (2–4). Restoring apoptosis is an important anticarcinogenic mechanism (2, 4, 5). Loss of apoptosis in colorectal cancer cells is linked to down-regulation in 15-lipoxygenase-1 (15-LOX-1) expression, and the primary product of 15-LOX-1 acting on linoleic acid, 13-S-hydroxyoctadecadienoic acid (13-S-HODE) (6), restores apoptosis in colorectal cancer cells (7). Nonsteroidal antiinf lammatory drugs (NSAIDs) induce apoptosis in colorectal cancer cells (8–10). We found previously that NSAIDs transcriptionally up-regulate the expression of 15-LOX-1 to induce apoptosis in colorectal cancer cells (11–13). These findings led us to search for downstream receptors involved in 15-LOX-1-induced apoptosis, none of which were identified previously. Peroxisome proliferatoractivated receptors (PPARs) can act as nuclear receptors for polyunsaturated fatty acids (arachidonic and linoleic acids) and their metabolites (14). PPAR-δ expression promotes colonic tumorigenesis (15). He et al. (16) found that NSAIDs suppress PPAR-δ activity in colon cancer cells (by interfering with PPAR-δ binding to DNA) as early as 10 h after treatment. These investigators speculated that the effects of NSAIDs on PPAR-δ and fatty acid metabolism may be linked (16). Because 13-S-HODE is a critical mediator of NSAID-induced apoptosis in colorectal cancer cells (11, 12), and linoleic acid can bind PPAR-δ (17), the present study investigated whether 13-S-HODE is the link between these two NSAID effects.
Materials and Methods
Materials. We obtained DLD-1 (colon cancer) and RKO (rectal cancer) cells, sulindac, NS-398, sulindac sulfone, and indomethacin as described (11, 12). Celecoxib was obtained from LKT Laboratories (St. Paul, MN), and rabbit anti-human PPAR-δ antibody was obtained from Santa Cruz Biotechnology. The HCT-116 parental PPAR-δ WT (PPAR-δ +/+) colon cancer cell line, HCT-116 PPAR-δ-null cell line (PPAR-δ –/–) (15), and PPAR-δ DNA-response element (DRE) reporter vector were gifts from Bert Vogelstein (The Johns Hopkins University School of Medicine, Baltimore). pMH-100-TK-luc reporter and Gal4-PPAR-δ ligand-binding domain (LBD) plasmids were provided by Ronald M. Evans (The Salk Institute, La Jolla, CA). Rabbit polyclonal anti-serum to recombinant human 15-LOX-1 was a gift from Mary Mulkins and Elliot Sigal (Roche Bioscience) and was also generated by Lampire Biological Laboratories (Pipersville, PA) by injecting the recombinant human 15-LOX-1 protein (provided by Mary Mulkins and Elliot Sigal) into rabbits. We obtained 13-S-HODE and linoleic acid (formulated in DMSO) from Cayman Chemical (Ann Arbor, MI). Other reagents, molecular-grade solvents, and chemicals were obtained either from commercial manufacturers or as specified below.
Cell Culture. HCT-116 cells were grown in McCoy's 5A medium (modified) containing 10% FBS and 1% penicillin/streptomycin (15). DLD-1 and RKO cells were grown in RPMI medium 1640 (11, 12). Cells were treated with: sulindac, indomethacin, and sulindac sulfone as described (11, 12); celecoxib, concentration 12.5 μM; and 1.35–13.5 μM 13-S-HODE or linoleic acid as described (12).
PPAR-δ Ligand-Binding Reporter Assays. Cells were transfected in 24-well plates with 0.4 μg of pMH-100-TK-luc per well, 0.4 μg of GAL4-PPAR-δ LBD per well (18), 0.2 μg of pSV-βgal [which encodes β-galactosidase (β-gal)] per well, and 3 μl of Lipofectamine 2000 (Invitrogen) per well and incubated overnight. 13-S-HODE or linoleic acid was added at 13.5 μM, and cells were cultured for 6 h. Control experiments used DMSO (the vehicle for 13-S-HODE and linoleic acid) only in a volume equal to the highest volume used for 13-S-HODE and linoleic acid (≤0.3%). Cells were harvested and lysed, and luciferase activity was measured by using a luciferase assay kit (Promega). Luciferase activity levels were normalized to the relative β-gal activity as measured by using a commercial kit (Invitrogen).
Transfection with a 15-LOX-1 Expression Vector. 15-LOX-1 cDNA was subcloned from a 15-LOX-1 cDNA-carrying pIND vector (Invitrogen) [provided by Joseph Cornicelli (Parke-Davis)] into a pAdenoVator-CMV5 (cytomegalovirus)-IRES (internal ribosomal entry segment)-GFP vector (Qbiogene) and used for transient transfections (with Lipofectamine 2000) into RKO and DLD-1 cells.
PPAR-δ Activation Assays. To measure PPAR-δ activation levels, we used a luciferase reporter construct containing PPAR-δ DRE in pBV-luc vector (16), which was transfected into cells with Lipofectamine 2000. Cells were treated with 13-S-HODE or linoleic acid (13.5 μM), and luciferase activity was measured as described above for the PPAR-δ ligand-binding reporter assays.
Scintillation Proximity Assays of 13-S-HODE Binding to PPAR-δ. The PPAR-δ LBDs, expressed in Escherichia coli as polyhistidinetagged fusion proteins, were purified, biotinylated, and immobilized on streptavidin-modified scintillation proximity assay beads (19). The radioligand for PPAR-δ was [3H]GW362433X. The assay buffer was 50 mM Hepes (pH 7)/50 mM KCl/5 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/0.1 mg/ml BSA/10 mM DTT. Increasing concentrations of 13-S-HODE were incubated in samples for at least 1 h at room temperature before the bound reactivity for each well was determined in a 1450 Microbeta Plus liquid scintillation counter (Wallac, Gaithersburg, MD).
Northern Blot Analysis of PPAR-δ RNA Expression. Total RNA was isolated, and Northern blot analyses were performed as described (13). The PPAR-δ probe was a 471-bp PPAR-δ cDNA fragment generated by RT-PCR using human liver mRNA (Clontech) with primers 5′-AGC-AGC-CTC-TTC-CTC-AAC-GAC-CAG-3′ and 5′-GGT-CTC-GGT-TTC-GGT-CTT-CTT-GAT-3′ (20).
Western Blot Analysis of PPAR-δ and 15-LOX-1. Protein samples were prepared and subjected to SDS/PAGE under reducing conditions (12). After transfer, blots were probed with a solution of rabbit antibody to human 15-LOX-1 (1:2,000 dilution) or PPAR-δ (1:500 dilution) and analyzed by the enhanced chemiluminescence method (Amersham Biosciences), as described (12).
Supporting Information. For further details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Results
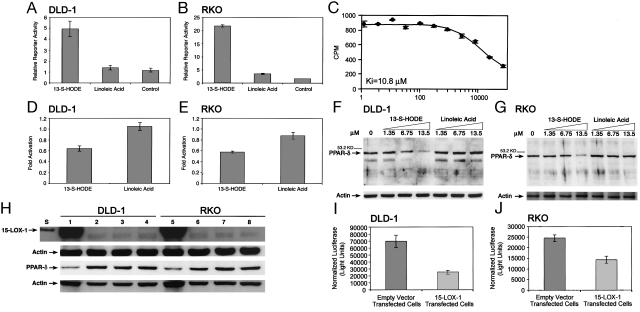
13-S-HODE Binds to PPAR-δ in Colorectal Cancer Cells. We used the Gal4-PPAR-δ LBD reporter assay to measure the ability of 13-S-HODE to bind to the PPAR-δ binding domain in RKO cells, which express cyclooxygenase (COX)-1 and -2, and in DLD-1 cells, which do not express COX-1 or -2. 13-S-HODE was 3.6- to 6.1-fold stronger than was the identical concentration of linoleic acid in activating the PPAR-δ LBD (Fig. 1 A and B) (13-S-HODE vs. linoleic acid and control, P < 0.0001 for RKO and P = 0.0012 for DLD-1). By using scintillation proximity assays, we found that 13-S-HODE binds directly to PPAR-δ with a Ki of 10.8 μM (Fig. 1C).
Fig. 1.
Effects of 15-LOX-1 and 13-S-HODE on PPAR-δ expression and activation in colorectal cancer cells. (A and B) Effects of 13-S-HODE on the PPAR-δ LBD in DLD-1 (A) and RKO (B) cells. DLD-1 and RKO cells were treated with 13.5 μM 13-S-HODE, linoleic acid, or an equivalent volume of vehicle solvent (DMSO, control). Luciferase activity for the Gal4-PPAR-δ LBD and pMH-100-TK-luc reporter plasmid system was measured and normalized to β-gal activity (relative to 100,000 β-gal units). Values shown are the means and SEMs of triplicate experiments. (C) Scintillation proximity assay of 13-S-HODE binding to PPAR-δ. Values on x axis are nM 13-S-HODE. (D and E) Effects of 13-S-HODE (13.5μM) on PPAR-δ activation. Luciferase activity for the DRE-luciferase reporter vector was measured and normalized toβ-gal activity (relative to 100,000 β-gal units). Values shown are the fold activation relative to control (DMSO-treated cells) and represent the means of triplicate experiments. Error bars represent SEM. (F and G) Effects of 13-S-HODE on PPAR-δ expression. Western blot analyses for PPAR-δ expression in cells treated with 13-S-HODE or linoleic acid, cultured for 24 h, and then harvested; repeated experiments showed similar results. Control cells (0) were treated with DMSO only. Equal loading was assessed by probing for actin. (H) Effects of 15-LOX-1 expression on PPAR-δ expression. RKO and DLD-1 cells were transfected with 15-LOX-1 expression vector, empty vector, or transfection media alone (mock transfection). Western blots show the expression of 15-LOX-1 and PPAR-δ in cells harvested at 24 h. Similar results were observed at 48 h and with repeated experiments. Lanes: S, standard 15-LOX-1 recombinant protein; 1 and 5, 15-LOX-1 expression vector transfected cells; 2 and 6, empty vector-transfected cells; 3 and 7, mock transfections; 4 and 8, cells without transfections. (I and J) Effects of 15-LOX-1 expression on PPAR-δ activity. DLD-1 (I) and RKO (J) cells were transfected with 15-LOX-1 expression vector or the empty vector and with DRE-luciferase reporter and pSV-βgal vectors. Luciferase activity values were normalized toβ-gal activity. Shown values are means and SEMs from 48-h experiments. Repeated experiments at 24 and 48 h showed similar results. 15-LOX-1 expression reduced PPAR-δ activation significantly compared (t test) with empty-vector-transfected RKO (E-RKO) (P = 0.0125) and empty-vector-transfected DLD-1 (P = 0.0052) cells.
13-S-HODE Down-Regulates PPAR-δ Expression and Activation in Colorectal Cancer Cells. Compared with linoleic acid (control), 13-S-HODE reduced the activation of PPAR-δ by ≈40% in DLD-1 cells (10 h, P < 0.0001) (Fig. 1D), 44% in RKO cells (18 h, P < 0.0001) (Fig. 1E), and in a concentration-dependent manner in both cell lines (24 h). In DLD-1 cells, 13-S-HODE reduced the activation of PPAR-δ by 6.6% at 1.35 μM, 37% at 6.75 μM, 40% at 13.5 μM, 66% at 27 μM, and 74% at 54 μM; in RKO cells, 13-S-HODE reduced the activation of PPAR-δ by 6.1% at 1.35 μM, 34% at 6.75 μM, 45% at 13.5 μM, 63% at 27 μM, and 74% at 54 μM. We also found that 13-S-HODE down-regulated PPAR-δ protein expression in a concentration-dependent manner in DLD-1 and RKO cells (Fig. 1 F and G). In contrast, linoleic acid failed to decrease PPAR-δ expression (Fig. 1 F and G).
15-LOX-1 Expression Down-Regulates PPAR-δ Expression and Transcriptional Activity in Colorectal Cancer Cells. RKO and DLD-1 cells transfected with pAdenoVator-CMV5-IRES-GFP vector carrying 15-LOX-1 cDNA expressed 15-LOX-1, whereas cells transfected with empty pAdenoVator-CMV5-IRES-GFP vector or transfection medium alone (mock transfection) did not (Fig. 1H). 13-S-HODE levels in 15-LOX-1-transfected DLD-1 cells (6.14 ± 0.43 ng/μg of protein) were higher than in empty-vector-transfected (2.75 ± 0.31, P < 0.001), mock-transfected (2.37 ± 0.18, P < 0.001), or nontransfected (2.05 ± 0.08, P < 0.001) DLD-1 cells at 24 h posttransfection. Similar 13-S-HODE results occurred in RKO cells (data not shown). PPAR-δ expression was lower in 15-LOX-1-transfected cells than in empty-vector- or mock-transfected cells (Fig. 1H). PPAR-δ activity was significantly lower in 15-LOX-1-transfected than in empty-vector-transfected cells (Fig. 1 I and J).
NSAIDs Down-Regulate PPAR-δ Expression in Colorectal Cancer Cells. We examined several NSAIDs for their ability to alter PPAR-δ expression; we began with sulindac (a nonselective COX inhibitor) and celecoxib (a selective COX-2 inhibitor), which exhibit clinical chemopreventive activity in colonic tumorigenesis (21, 22). Sulindac and celecoxib reduced PPAR-δ RNA expression in RKO and DLD-1 cells at 48 h (Fig. 2 Top). Celecoxib reduced PPAR-δ protein expression in a time-dependent manner starting at 48 h posttreatment (Fig. 2 Middle). Similarly, other NSAIDs (NS-398, indomethacin, sulindac, and sulindac sulfone) down-regulated PPAR-δ protein expression (Fig. 2 Bottom).
Fig. 2.
NSAID effects on PPAR-δ expression in colorectal cancer cells. (Top) Effects of NSAIDs on PPAR-δ RNA expression in colorectal cancer cells. Equal RNA loading was assessed by probing for GAPDH. (Middle) Time course for the effects of celecoxib on PPAR-δ protein expression in DLD-1 cells. Similar results were observed with RKO cells (data not shown). (Bottom) Effects of NSAIDs on PPAR-δ protein expression in RKO cells. Cells were treated with NSAIDs, harvested 72 h later, processed for Western blotting, and probed with PPAR-δ antibody. Lanes: 1, control; 2, NS-398; 3, indomethacin; 4, sulindac; 5, sulindac sulfone. Similar results were observed with DLD-1 cells (data not shown) and with repeated experiments.
15-LOX-1 Up-Regulation, PPAR-δ Down-Regulation, and NSAIDInduced Apoptosis. Given the temporal relationship between NSAID up-regulation of 15-LOX-1 (at 24 h) (11, 12) and down-regulation of PPAR-δ (at 48 h), we examined the possible mechanistic link between these cellular events by creating an in vitro system of RKO cells in which NSAID-induced 15-LOX-1 expression is blocked by 15-LOX-1-antisense (AS). Stable transfection of an AS construct for 15-LOX-1 blocked the ability of celecoxib to induce 15-LOX-1 expression (Fig. 3A) and suppressed the growth-inhibitory effects of celecoxib (data not shown) in four selected clones. Furthermore, celecoxib failed to reduce PPAR-δ activity in the two 15-LOX-1-AS clones (clones 3 and 4) tested for this effect. PPAR-δ activity with celecoxib treatment was 110 ± 4.7% (mean ± SEM) of PPAR-δ activity with DMSO (control) treatment in clone 3 and 98 ± 5.5% of PPAR-δ activity with DMSO treatment in clone 4. In contrast, celecoxib reduced PPAR-δ activity in pcDNA3.1 empty-vector-transfected RKO cells (E-RKO); PPAR-δ activity in celecoxib-treated cells was 66 ± 5.3% of the levels in DMSO-treated cells at 48 h. The differences between the effects of celecoxib on PPAR-δ activity in E-RKO versus 15-LOX-1-AS-transfected clones were statistically significant (P < 0.0125). In contrast, the effects of celecoxib on PPAR-δ activity were not statistically different between E-RKO and W-RKO. We selected the stably transfected AS 15-LOX-1 clone 4 (15-LOX-1-AS4-RKO) to further characterize the effects that blocking 15-LOX-1 expression would have on the response of RKO cells to celecoxib in vitro. Celecoxib failed to up-regulate 15-LOX-1 or down-regulate PPAR-δ expression in 15-LOX-1-AS4-RKO cells cultured as long as 240 h after celecoxib treatment (Fig. 3B). In contrast, celecoxib up-regulated 15-LOX-1 and down-regulated PPAR-δ in W-RKO and E-RKO (Fig. 3B). Celecoxib increased 13-S-HODE levels (by a mean of 2.57-fold in nine experiments) in W-RKO (vs. controls) 48 h after treatment (P < 0.0001). The level of celecoxib-increased 13-S-HODE was inhibited by >50% in 15-LOX-1-AS4-RKO cells (P = 0.0006 in nine experiments), compared with levels in celecoxib-treated E-RKO and W-RKO. 13-S-HODE levels in celecoxib-treated E-RKO and W-RKO were similar (P = 0.14 in nine experiments).
Fig. 3.
Effects of celecoxib-induced 15-LOX-1 expression on PPAR-δ expression and apoptosis. (A) Effects of stable transfection of 15-LOX-1-AS on celecoxibinduced 15-LOX-1 in RKO cells. S, standard positive control of human 15-LOX-1 recombinant protein; –, control cells that were treated with DMSO (celecoxib solvent); +, celecoxib-treated cells; Empty vector, empty-vector-transfected cells; 1–4, AS-transfected clones. (B) Effects of celecoxib on 15-LOX-1 and PPAR-δ in stably transfected RKO cells with 15-LOX-1-AS clone 4 in protracted cell-culture exposure (240 h after celecoxib treatment). Similar results were observed with AS clone 3 (data not shown). (C) Effects of blocking 15-LOX-1-induced expression by celecoxib on growth inhibition by celecoxib. Growth ratios of celecoxib (cele)treated WT RKO (W-RKO), E-RKO, and 15-LOX-1-AS clone 4 stably transfected cells (15-LOX-1-AS) to control cells (DMSO-treated only) at 72 h. Mean ± SEM of triplicate experiments. (cele, 15-LOX-1-AS vs. cele, W-RKO, P = 0.0008; cele, 15-LOX-1-AS vs. cele, E-RKO, P = 0.0007; cele, W-RKO vs. cele, E-RKO, P = 0.88). (D) Effects of blocking 15-LOX-1 expression on celecoxib-induced apoptosis measured by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay in RKO cells. Percentages of TUNEL-positive cells in W-RKO, E-RKO, and 15-LOX-1-AS cells are means ± SEM of triplicate experiments at 72 h after celecoxib or DMSO (control) treatment. (cele, 15-LOX-1-AS vs. cele, W-RKO, P < 0.0001; cele, 15-LOX-1-AS vs. cele, E-RKO, P < 0.0001). (E) Effects of blocking 15-LOX-1 expression on celecoxib-induced apoptosis as measured by sub-G1 fractions in RKO cells. Sub-G1 fractions in W-RKO, E-RKO, and 15-LOX-1-AS treated with either celecoxib or DMSO (control) and harvested 72 h later are means ± SEM of triplicate experiments. (cele, 15-LOX-1-AS vs. cele, W-RKO, P = 0.0015; cele, 15-LOX-1-AS vs. cele, E-RKO, P < 0.0001). (F) Effects of blocking 15-LOX-1 expression on celecoxib-induced apoptosis as measured by DNA laddering assay. Lanes: 1, standard DNA ladder; 2, W-RKO cells; 3, W-RKO cells treated with celecoxib; 4, E-RKO cells; 5, E-RKO cells treated with celecoxib; 6, 15-LOX-1-AS (control); 7, 15-LOX-1-AS cells treated with celecoxib.
To further study the link between 15-LOX-1 up-regulation, PPAR-δ down-regulation, and NSAID-induced apoptosis, we measured the effects of celecoxib on apoptosis in W-RKO, 15-LOX-1-AS4-RKO, and E-RKO. Celecoxib's ability to inhibit growth and induce apoptosis (measured by TUNEL, sub-G1, and DNA laddering assays) was markedly lower in 15-LOX-1-AS4-RKO than in W-RKO or E-RKO (Fig. 3 C–F).
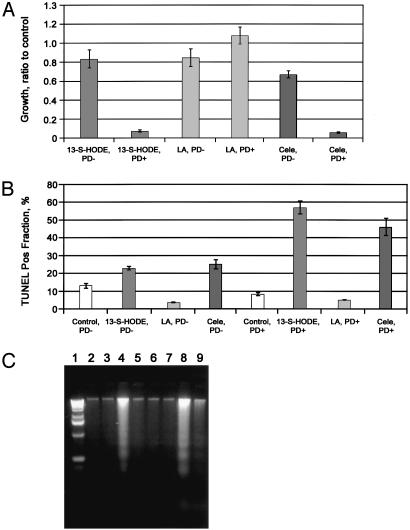
PPAR-δ-Knock-Out Suppresses Apoptosis Induction by Celecoxib and 13-S-HODE. We next evaluated the role of PPAR-δ in apoptosis induction by celecoxib or 13-S-HODE in an experimental PPAR-δ-knock-out model in HCT-116 colon cancer cells. Celecoxib inhibited cell growth and induced apoptosis in WT HCT-116 cells, which express PPAR-δ, whereas these effects were markedly suppressed in PPAR-δ-null HCT-116 cells (Fig. 4). Parallel to these findings, 13-S-HODE inhibition of cell growth and induction of apoptosis were markedly diminished in PPAR-δ-null vs. WT HCT-116 cells (Fig. 4). Linoleic acid failed to show significant growth-inhibitory or apoptotic effects in either cell line.
Fig. 4.
PPAR-δ effects on celecoxib and 13-S-HODE-induced apoptosis in colon cancer cells. (A) Growth ratios of treated cells represent the number of viable cells divided by the number of viable cells in the control experiment (DMSO-treated only) at 72 h. PD+, WT HCT-116 cells that express PPAR-δ;PD–, HCT-116 cells that are PPAR-δ null. 13-S-HODE and linoleic acid (LA) concentrations were 13.5 μM. Means ± SEMs of triplicate experiments are shown. (13-S-HODE, PD–, vs. 13-S-HODE, PD+, P < 0.0001; celecoxib, PD–, vs. celecoxib, PD+, P < 0.003.) Repeated experiments showed similar results. (B) Effects of silencing PPAR-δ expression on apoptosis induction by 13-S-HODE and celecoxib, measured by TUNEL assay. Means ± SEMs of triplicate experiments are shown. (13-S-HODE, PD– vs. 13-S-HODE, PD+, P < 0.0001; celecoxib, PD– vs. celecoxib, PD+, P < 0.001; linoleic acid, PD– vs. linoleic acid, PD+, P = 0.672.) Similar results were found with sub-G1 fraction assays and in triplicate experiments (data not shown). (C) Effects (measured by DNA laddering) of silencing PPAR-δ expression on apoptosis induction by celecoxib and 13-S-HODE. Lanes: 1, standard DNA ladder; 2, PD+ cells treated with DMSO only (control); 3, PD– cells treated with DMSO only (control); 4, PD+ cells treated with 13-S-HODE; 5, PD– cells treated with 13-S-HODE; 6, PD+ cells treated with linoleic acid; 7, PD– cells treated with linoleic acid; 8, PD+ cells treated with celecoxib; 9, PD– cells treated with celecoxib.
Celecoxib Effects on 15-LOX-1, PPAR-δ, and Tumorigenesis in Vivo. To examine the in vivo relevance of our in vitro findings, we tested the mechanistic link between celecoxib's induction of 15-LOX-1 expression, down-regulation of PPAR-δ, and inhibition of tumorigenesis in a nude mouse model with xenografts of WT-RKO and RKO cells transfected with 15-LOX-1-AS or empty vector. Celecoxib significantly inhibited tumorigenesis in RKO cells transfected with pcDNA3.1 empty vector (Fig. 5A) and in W-RKO (data not shown). In contrast, the antitumorigenic effects of celecoxib were blocked in RKO cells that were stably transfected with 15-LOX-1-AS clones 3 (Fig. 5B) and 4 (Fig. 5C). The tumor growth rates in the non-celecoxib-treated animal groups (Fig. 5 A–C, solid lines) were variable and inconsistent with specific 15-LOX-1-AS effects. Celecoxib up-regulated 15-LOX-1 and down-regulated PPAR-δ protein expression in W-RKO (Fig. 5D) and empty-vectortransfected cells (data not shown) but not in cells stably transfected with 15-LOX-1-AS clone 3 or 4 (Fig. 5D).
Fig. 5.
Effects of celecoxib-induced 15-LOX-1 expression on PPAR-δ expression and tumorigenesis in vivo. Wild type RKO (W RKO) and RKO cells transfected with 15-LOX-1-AS clone 3 (15-LOX AS-3), 15-LOX-1-AS clone 4 (15-LOX AS-4), and E-RKO were grown as xenografts in nude mice. Animals were randomized to celecoxib treatment or a control diet. Celecoxib inhibited the growth of E-RKO (A) but not 15-LOX AS-3 (B) or 15-LOX AS-4 (C) cells. (D) 15-LOX-1 and PPAR-δ Western blot analyses of W-RKO, 15-LOX AS-3, and 15-LOX AS-4 tissue xenografts. Lanes: S, standard 15-LOX-1 recombinant protein; 1, W-RKO (WT); 2, W-RKO + celecoxib; 3, 15-LOX AS-3 (AS-3); 4, 15-LOX AS-3 + celecoxib; 5, 15-LOX AS-4 (AS-4); 6, 15-LOX AS-4 + celecoxib. Actin expression was used to assess equal loading. (E) Proposed model of 15-LOX-1, 13-S-HODE, and PPAR-δ as a signaling pathway that can be modulated by NSAIDs to induce apoptosis and inhibit colorectal tumorigenesis.
Discussion
We found that (i) 13-S-HODE, the main product of 15-LOX-1, bound to PPAR-δ, suppressed PPAR-δ activation, and down-regulated PPAR-δ expression; (ii) NSAIDs induced 15-LOX-1 expression to down-regulate PPAR-δ expression and induce apoptosis; and (iii) PPAR-δ is an important signaling receptor for both NSAIDs and 13-S-HODE for inducing apoptosis. These results demonstrate the binding of 13-S-HODE to PPAR-δ and the biological effects of this binding on PPAR-δ expression and activation. The role of 13-S-HODE in suppressing PPAR-δ expression is important because of the recently recognized role of PPAR-δ in promoting colonic tumorigenesis (15, 16, 23). Both exogenous and endogenous 13-S-HODE (the latter formed after 15-LOX-1 transfection) down-regulated PPAR-δ expression and activity. 13-S-HODE bound to PPAR-δ, decreased PPAR-δ activation, and down-regulated PPAR-δ expression at the same concentration that we previously showed to induce apoptosis in colon cancer cells (12); these results suggest that these biological effects of 13-S-HODE are linked. This study found that these effects are mechanistically linked in PPAR-δ-null cancer-cell experiments, showing that the loss of PPAR-δ expression markedly suppressed apoptosis induction by 13-S-HODE. Linoleic acid, the parent compound of 13-S-HODE, failed to exert similar effects, which indicates that these effects are specific to 13-S-HODE. Our results, therefore, define a previously unknown signaling pathway for inducing apoptosis in colorectal cancer cells: the metabolism of linoleic acid by 15-LOX-1 leads to 13-S-HODE production, which down-regulates PPAR-δ expression and activity to induce apoptosis (Fig. 5E).
In addition to defining an apoptotic pathway involving 15-LOX-1 and PPAR-δ signaling, we have demonstrated that this pathway can be therapeutically modulated (by NSAIDs) to induce apoptosis. A link during apoptosis induction between LOX metabolism and NSAID–PPAR-δ signaling was postulated (16) but never confirmed. Our prior studies, which showed that 15-LOX-1-induced expression by NSAIDs is crucial to NSAID-induced apoptosis, led us to investigate the existence of a mechanistic link to PPAR-δ.We hypothesized, based on our previous findings that NSAIDs upregulate 15-LOX-1 starting at 24 h, that if NSAIDs affect PPAR-δ expression through a 15-LOX-1 signaling pathway, this effect on PPAR-δ would occur after 24 h posttreatment (11, 12). Other prior findings that NSAIDs had no effect on PPAR-δ RNA expression for as long as 36 h after treatment (16) supported this prediction. Indeed NSAIDs down-regulated PPAR-δ expression at 48 h posttreatment in the present study, which was consistent with our hypothesis. These findings indicated the temporal relationship between NSAID upregulation of 15-LOX-1 and down-regulation of PPAR-δ.
We used a 15-LOX-1-AS model to investigate whether the temporally associated NSAID effects of up-regulated 15-LOX-1, down-regulated PPAR-δ (by 13-S-HODE), and apoptosis induction are linked mechanistically. The 15-LOX-1-AS construct blocked celecoxib-induced 15-LOX-1 expression, which in turn inhibited celecoxib from forming 13-S-HODE, inducing apoptosis, and inhibiting cell growth. Furthermore, blocking 15-LOX-1 prevented PPAR-δ down-regulation by celecoxib, thus indicating the mechanistic link between 15-LOX-1 up-regulation, PPAR-δ down-regulation, and apoptosis induction by NSAIDs. We extended these in vitro findings to the in vivo setting of a nude mouse xenograft model. Our findings in this model established the physiological relevance of the mechanistic link between celecoxib induction of 15-LOX-1 expression, down-regulation of PPAR-δ, and inhibition of tumorigenesis. In WT or empty vector-transfected xenografts, celecoxib up-regulated 15-LOX-1 expression, down-regulated PPAR-δ, and inhibited tumorigenesis. These celecoxib effects were blocked in xenografts in which the expression of 15-LOX-1 was blocked by a stably transfected 15-LOX-1-AS construct. Therefore, 15-LOX-1 expression is crucial to celecoxib's ability to down-regulate PPAR-δ and inhibit tumorigenesis.
Based on findings in a colon-cancer cell system with overexpressed PPAR-δ, He et al. (16) concluded that reducing PPAR-δ activity is an important mechanism of NSAID-induced apoptosis. Park et al. (15) (of the same group) subsequently found that tumorigenesis was inhibited in xenografts of PPAR-δ-null HCT-116 colon cancer cells, consistent with the findings of He et al.; they also found, however, that high concentrations of sulindac sulfide (≥80 μM) inhibited growth of PPAR-δ-null HCT-116 cells in vitro, which raised a question about whether the suppression of PPAR-δ activity is crucial to growth inhibition by NSAIDs. The NSAID–PPAR-δ relationship was examined also by another group [Peters et al. (24)], who showed in a PPAR-δ-knock-out mouse model that PPAR-δ is an important signaling receptor for the chemopreventive effects of sulindac. The difference between sulindac sulfide/sulindac effects in the in vitro (15) and in vivo (24) PPAR-δ-knock-out models may relate to the high concentration of sulindac sulfide used in the in vitro model (15). Therefore, we tested celecoxib at a markedly lower concentration (12.5 μM), which would be closer to achievable pharmacological levels in humans. Apoptosis induction and growth inhibition by celecoxib were decreased significantly in PPAR-δ-null cells, indicating that PPAR-δ is an important signaling receptor involved with NSAID-induced apoptosis.
Prostaglandin I2 (PGI2) a COX-2 product, activates PPAR-δ in human colon cancers (23), suggesting that inhibiting COX-2 might help suppress PPAR-δ activation. He et al. (16) observed that the NSAID sulindac sulfone, which is devoid of COX-inhibitory activity, inhibited PPAR-δ activity in the colon cancer cell line HCT-116, which lacks COX-2 expression; this finding suggested that NSAID modulation of PPAR-δ activity is independent of COX-2 inhibition. We previously found that sulindac sulfone induces 15-LOX-1 expression to trigger apoptosis in DLD-1 colon cancer cells, which lack the expression of both COX-2 and COX-1 (12). In the current study, we found that NSAIDs (including sulindac sulfone) and 13-S-HODE down-regulate PPAR-δ expression in DLD-1 cells. These findings indicate that NSAIDs can down-regulate PPAR-δ expression independently of COX-2 inhibition by modulation of the 15-LOX-1 pathway to form 13-S-HODE. PGI2 activation of PPAR-δ may be relevant in cells expressing COX-2, and further studies to elucidate the relative effects of the various lipid metabolic pathways on PPAR-δ are warranted in cells expressing COX-2.
Our current findings demonstrate the biological effects of 13-S-HODE on PPAR-δ expression and activation; they demonstrate also the ability of NSAIDs to modulate PPAR-δ expression and activity by 15-LOX-1 up-regulation, which is a signaling pathway for restoring apoptosis and inhibiting tumorigenesis in colorectal cancer cells. Additional studies are needed to define the role of this signaling pathway in tumorigenesis further. Future interventions that target this signaling pathway as a means of therapeutically restoring apoptosis in colorectal cells during tumorigenesis may lead to novel and improved agents for treating and preventing colorectal cancer.
Supplementary Material
Acknowledgments
We thank Deborah Cohen for assistance with data analyses and Donging Chen and Boaxing Gaun for technical assistance. This work was supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services Grants 1KO7 CA86970 and CA16672, and a grant from the Cancer Research Foundation of America.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 13-S-HODE, 13-S-hydroxyoctadecadienoic acid; 15-LOX-1, 15-lipoxygenase-1; AS, antisense; β-gal, β-galactosidase; COX, cyclooxygenase; E-RKO, pcDNA3.1 empty-vector-transfected RKO cells; LBD, ligand-binding domain; NSAIDs, nonsteroidal antiinflammatory drugs; PPARs, peroxisome proliferator-activated receptors; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; W-RKO, WT RKO cells.
References
- 1.Hall, P. A., Coates, P. J., Ansari, B. & Hopwood, D. (1994) J. Cell Sci. 107, 3569–3577. [DOI] [PubMed] [Google Scholar]
- 2.Evan, G. I. & Vousden, K. H. (2001) Nature 411, 342–348. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson, I. P. & Bodmer, W. F. (1995) Proc. Natl. Acad. Sci. USA 92, 11130–11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone, R. W., Ruefli, A. A. & Lowe, S. W. (2002) Cell 108, 153–164. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson, D. W. (2000) Nature 407, 810–816. [DOI] [PubMed] [Google Scholar]
- 6.Brash, A. R., Boeglin, W. E. & Chang, M. S. (1997) Proc. Natl. Acad. Sci. USA 94, 6148–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shureiqi, I., Wojno, K. J., Poore, J. A., Reddy, R. G., Moussalli, M. J., Spindler, S. A., Greenson, J. K., Normolle, D., Hasan, A. A., Lawrence, T. S. & Brenner, D. E. (1999) Carcinogenesis 20, 1985–1995. [DOI] [PubMed] [Google Scholar]
- 8.Piazza, G. A., Rahm, A. L., Krutzsch, M., Sperl, G., Paranka, N. S., Gross, P. H., Brendel, K., Burt, R. W., Alberts, D. S., Pamukcu, R., et al. (1995) Cancer Res. 55, 3110–3116. [PubMed] [Google Scholar]
- 9.Shiff, S. J., Qiao, L., Tsai, L. L. & Rigas, B. (1995) J. Clin. Invest. 96, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samaha, H. S., Kelloff, G. J., Steele, V., Rao, C. V. & Reddy, B. S. (1997) Cancer Res. 57, 1301–1305. [PubMed] [Google Scholar]
- 11.Shureiqi, I., Chen, D., Lee, J. J., Yang, P., Newman, R. A., Brenner, D. E., Lotan, R., Fischer, S. M. & Lippman, S. M. (2000) J. Natl. Cancer Inst. 92, 1136–1142. [DOI] [PubMed] [Google Scholar]
- 12.Shureiqi, I., Chen, D., Lotan, R., Yang, P., Newman, R. A., Fischer, S. M. & Lippman, S. M. (2000) Cancer Res. 60, 6846–6850. [PubMed] [Google Scholar]
- 13.Shureiqi, I., Jiang, W., Fischer, S. M., Xu, X., Chen, D., Lee, J. J., Lotan, R. & Lippman, S. M. (2002) Cancer Res. 62, 1178–1183. [PubMed] [Google Scholar]
- 14.Chawla, A., Repa, J. J., Evans, R. M. & Mangelsdorf, D. J. (2001) Science 294, 1866–1870. [DOI] [PubMed] [Google Scholar]
- 15.Park, B. H., Vogelstein, B. & Kinzler, K. W. (2001) Proc. Natl. Acad. Sci. USA 98, 2598–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, T. C., Chan, T. A., Vogelstein, B. & Kinzler, K. W. (1999) Cell 99, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, H. E., Lambert, M. H., Montana, V. G., Parks, D. J., Blanchard, S. G., Brown, P. J., Sternbach, D. D., Lehmann, J. M., Wisely, G. B., Willson, T. M., et al. (1999) Mol. Cell 3, 397–403. [DOI] [PubMed] [Google Scholar]
- 18.Kliewer, S. A., Umesono, K., Noonan, D. J., Heyman, R. A. & Evans, R. M. (1992) Nature 358, 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols, J. S., Parks, D. J., Consler, T. G. & Blanchard, S. G. (1998) Anal. Biochem. 257, 112–119. [DOI] [PubMed] [Google Scholar]
- 20.Guan, Y., Zhang, Y., Davis, L. & Breyer, M. D. (1997) Am. J. Physiol. 273, F1013–F1022. [DOI] [PubMed] [Google Scholar]
- 21.Giardiello, F. M., Hamilton, S. R., Krush, A. J., Piantadosi, S., Hylind, L. M., Celano, P., Booker, S. V., Robinson, C. R. & Offerhaus, G. J. (1993) N. Engl. J. Med. 328, 1313–1316. [DOI] [PubMed] [Google Scholar]
- 22.Steinbach, G., Lynch, P. M., Phillips, R. K., Wallace, M. H., Hawk, E., Gordon, G. B., Wakabayashi, N., Saunders, B., Shen, Y., Fujimura, T., et al. (2000) N. Engl. J. Med. 342, 1946–1952. [DOI] [PubMed] [Google Scholar]
- 23.Gupta, R. A., Tan, J., Krause, W. F., Geraci, M. W., Willson, T. M., Dey, S. K. & DuBois, R. N. (2000) Proc. Natl. Acad. Sci. USA 97, 13275–13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters, J. M., Lee, S. S., Li, W., Ward, J. M., Gavrilova, O., Everett, C., Reitman, M. L., Hudson, L. D. & Gonzalez, F. J. (2000) Mol. Cell. Biol. 20, 5119–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.