Abstract
The sympathetic nervous system is designed to respond to stress. Adenylyl cyclase (AC) is the keystone of sympathetic transmission, yet its role in response to acute overload in the heart or in the pathogenesis of heart failure is controversial. We examined the effects of pressure overload, induced by thoracic aortic banding, in mice in which type 5 AC, a major cardiac AC isoform, was disrupted (AC5–/–). Left ventricular weight/tibial length ratio (LVW/TL) was not different between the WT and AC5–/– at baseline and increased progressively and similarly in both groups at 1 and 3 wk after aortic banding. However, LV ejection fraction (LVEF) fell in WT at 3 wk after banding (from 70 ± 2.8 to 57 ± 3.9%, P < 0.05), and this decrease was associated with LV dilatation, indicating incipient cardiac failure. In contrast, AC5–/– mice did not exhibit a fall in LVEF from 74 ± 2.2%. The number of apoptotic myocytes was similar at baseline, but it increased roughly 4-fold in WT at both 1 and 3 wk after banding, and significantly less, P < 0.05, in AC5–/–. Importantly, the increase in apoptosis occurred before the decline in LVEF in WT. The protective mechanism seems to involve Bcl-2, which was up-regulated significantly more in AC5–/– mice with pressure overload. Our findings suggest that limiting type 5 AC plays a protective role in response to pressure overload and the development of heart failure, potentially through limiting the incidence of myocardial apoptosis.
Heart failure is a major cause of death and disability in the U.S., and chronic pressure overload is a common cause of heart failure in patients (1). In response to pressure overload, the myocardium undergoes adaptive hypertrophy to maintain cardiac output against the increased afterload. Although this adaptation is initially beneficial, prolonged pressure overload eventually leads to the development of heart failure, as reflected by the dilatation of the left ventricle (LV) and a decrease in cardiac contractility, e.g., left ventricular ejection fraction (LVEF; ref. 2). Pressure overload also results in apoptosis, which could be part of the mechanism of cardiac decompensation (3).
The role of β-adrenergic receptor (β-AR) signaling is well defined as a primary defense against acute stress or changes in hemodynamic load; however, its role in the pathogenesis of heart failure, although studied extensively, remains controversial (4, 5). Recent studies suggest that β-AR blockade, rather than stimulation, is beneficial in patients with heart failure (5–7). Most prior experimental studies on this topic have focused on mouse models overexpressing β-AR (8–11), Gsα (12–14), or protein kinase A (PKA) (15), and the results from these studies support the use of β-AR blockade in heart failure, because these genetically engineered mouse models demonstrated that prolonged β-AR stimulation results in cardiomyopathy and that the adverse effects of pressure overload are exacerbated (11). Interestingly, the results from genetically engineered mouse models in which isoforms of adenylyl cyclase (AC) have been overexpressed are at variance with the above results (16–22). AC plays a pivotal role in β-AR signaling, transducing the signal generated from coupling of the ligand, most commonly norepinephrine, with the β-AR and Gsα to catalyze cAMP, which consequently modulates the L-type Ca2+ channel and augments cardiac contractility. In part, differences in results can be ascribed to different isoforms of AC, which are expressed in the heart. There are nine major mammalian isoforms of AC, with type 6 AC being the major fetal cardiac AC isoform, and type 5 AC, the major cardiac isoform in the adult (23, 24). Overexpressing type 6 AC results in maintained cardiac function and no myocardial fibrosis, even in 19-mo-old mice (20). Furthermore, overexpression of type 6 AC in overexpressed Gq mice, which normally develop cardiomyopathy, rescued cardiac function (18, 19). Similarly, when type 5 AC-overexpressed mice were mated with Gq mice, cardiac function was restored (16). Thus, the results from these studies suggest that overexpressing AC in the heart is potentially beneficial in the pathogenesis of heart failure.
A better experimental design would examine the effects of pressure overload in an animal model with disruption of a major AC isoform because this experimental design is more mechanistic than overexpression of a gene. Until this time, this experiment has not been possible. Recently, we developed a mouse model in which type 5 AC was disrupted (AC5–/–; ref. 25). In this animal model, cAMP-PKA signaling was diminished in the heart by ≈30–40%. The goal of the current investigation was to examine the effects of chronic pressure overload induced by aortic banding in AC5–/– and WT controls. We specifically examined the extent to which LV hypertrophy and apoptosis developed in response to pressure overload and the resultant effects on cardiac function. We then determined whether the increased apoptosis after chronic pressure overload preceded or coincided with the decline in LV function and, finally, examined alterations in molecular signaling that would potentially mediate the apoptosis.
Methods
Generation of Knockout Mice. The targeting construct was prepared by ligating a fragment from the 5′ end of the type 5 AC gene, which contains the exon with the first translation initiation site (5′ arm); a fragment containing a neomycin resistance gene driven by a phosphoglycerate kinase promoter; and a 7.0-kb fragment of the type 5 AC gene (3′ arm) into pBluescript II KS (Stratagene). Embryonic stem cells were transfected with 50 μg of linearized targeting vector by electroporation. Two clones were injected into C57BL/6 blastocysts, and chimeras were obtained. These chimeras successfully allowed germ-line transmission and were crossed with C57BL/6 females. F1-heterozygous offspring were then interbred to produce homozygous mutations. All mice were 129/SvJ-C57BL/6 mixed background littermates from F1 heterozygote crosses. All experiments were performed in 4- to 6-mo-old homozygous AC5–/– and WT littermates. This study was approved by the Animal Care and Use Committee at New Jersey Medical School.
Aortic Banding. Transverse aortic banding or sham operation was performed in 4- to 6-mo-old homozygous AC5–/– and WT littermates. The method of imposing pressure overload in mice has been described (26). Mice were anesthetized i.p. with a mixture of ketamine (0.065 mg/g), xylazine (0.013 mg/g), and acepromazine (0.002 mg/g). Mice were ventilated via intubation with a tidal volume of 0.2 ml and a respiratory rate of 110 breaths per minute. The left side of the chest was opened at the second intercostal space, and the transverse thoracic aorta was constricted. To measure the pressure gradient across the constriction, two high-fidelity catheter tip transducers (1.4 F; Millar Instruments, Houston) were used at 1 wk after aortic banding. One was inserted into the right carotid artery and the other into the right femoral artery, and they were advanced carefully to the ascending aorta and abdominal aorta, respectively, where pressures were measured simultaneously.
Echocardiography. Mice were anesthetized as noted above. Echocardiography was performed by using ultrasonography (Sequoia C256; Acuson, Malvern, PA; refs. 12–14). A dynamically focused 13-MHz annular array transducer was applied from below, by using a warmed saline bag as a standoff. M-mode measurements of LV internal diameter were made from more than three beats and averaged. Measurements of the LV end-diastolic diameter (LVEDD) were taken at the time of the apparent maximal LV diastolic dimension whereas measurements of the LV endsystolic diameter (LVESD) were taken at the time of the most anterior systolic excursion of the posterior wall. LVEF was calculated by the cubic method: LVEF (%) = [(LVEDD)3 – (LVESD)3]/(LVEDD)3.
Evaluation of Apoptosis. DNA fragmentation was detected in situ by using terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (14). Briefly, deparaffinized sections were incubated with proteinase K and DNA fragments labeled with biotin-conjugated dUTP and terminal deoxyribonucleotide transferase and visualized with FITC-ExtrAvidin (Sigma–Aldrich). Nuclear density was determined by manual counting of 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI)-stained nuclei in six fields of each animal by using the ×40 objective, and the number of TUNEL-positive nuclei was counted by examining the entire section by using the same power objective. Limiting the counting of total nuclei and TUNEL-positive nuclei to areas with a true cross section of myocytes made it possible to selectively count only those nuclei that were clearly within myocytes. For some samples, triple staining with propidium iodide (Vector Laboratories), TUNEL, and anti-α-sarcomeric actin antibody (Sigma–Aldrich), and subsequent analyses by using confocal microscopy, were performed to verify the results obtained with light microscopy.
Myocyte Cross-Sectional Area. The myocyte cross-sectional area was measured from images captured from silver-stained 1-μm-thick methacrylate sections (14). Suitable cross sections were defined as having nearly circular capillary profiles and circular-to-oval myocyte sections. No correction for oblique sectioning was made. The outline of 100–200 myocytes was traced in each section. metamorph image system software (Universal Imaging, Media, PA) was used to determine myocyte cross-sectional area.
Western Blotting. Crude cardiac membrane fractions were prepared and separated on 4–20% SDS-polyacrylamide gel and blotted onto nitrocellulose membrane (27). Western blotting was conducted with anti-Bcl-2 and anti-Bax antibodies (BD Biosciences). Expression of these proteins was quantified by densitometry.
RNase Protection Assay. Total RNA in the heart was prepared, and the amount of mRNA of Bcl-2 was determined by RNase protection assay by using RPA III kit (Ambion, Austin, TX). To probe Bcl-2, a partial fragment of mouse Bcl-2 gene was obtained by RT-PCR. A human 18S rRNA probe was used as an internal control. The relative intensity of Bcl-2 to 18S rRNA was quantified by densitometry.
Statistical Analysis. All data are reported as mean ± SEM. Comparisons between AC5–/– and WT values were made by using Student's t test. For statistical analysis of data from multiple groups, ANOVA was used. P < 0.05 was taken as a minimal level of significance.
Results
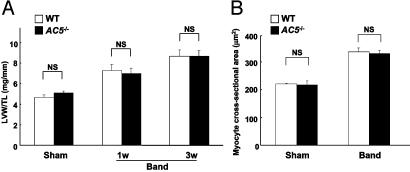
Disruption of Type 5 AC Did Not Affect the Development of Cardiac Hypertrophy. At baseline, there was no difference between WT and AC5–/– in the LV weight (LVW; mg)/tibial length (TL; mm) (WT 4.7 ± 0.2, AC5–/– 5.1 ± 0.2 mg/mm, n = 9–14, P = NS). The time course and the degree of the development of cardiac hypertrophy (LVW/TL) in response to pressure overload were similar between WT and AC5–/– (Fig. 1A). LVW/body weight, another index of cardiac hypertrophy, confirmed the data from LVW/TL (data not shown). Myocyte cross-sectional area, another index of hypertrophy, increased similarly in both WT and AC5–/– at 3 wk of banding, confirming the gross pathological data (Fig. 1B).
Fig. 1.
Comparison of cardiac hypertrophy after aortic banding in WT and AC5–/–. Transverse aortic banding or sham operation was applied to either WT or AC5–/–. (A) LVW (mg)/TL (mm) was determined at 1 and 3 wk. LVW/TL of sham-operated animals was obtained at 1 and 3 wk, and the data were combined. The degree of cardiac hypertrophy increased progressively at 1 and 3 wk but was similar in both WT and AC5–/– (n = 6for1wkand n = 8–10 for 3 wk). (B) Cardiac myocyte cross-sectional area was determined at 3 wk. There was also no significant difference in myocyte cross-sectional area between WT and AC5–/–. The fact that increases in LVW/TL were greater than increases in myocyte cross-sectional area with aortic banding can be ascribed to the fact that cross-sectional area assesses only two of three dimensions of myocyte size (increases in thickness are not measured) (n = 4–5 each for sham and banded). NS, not significant.
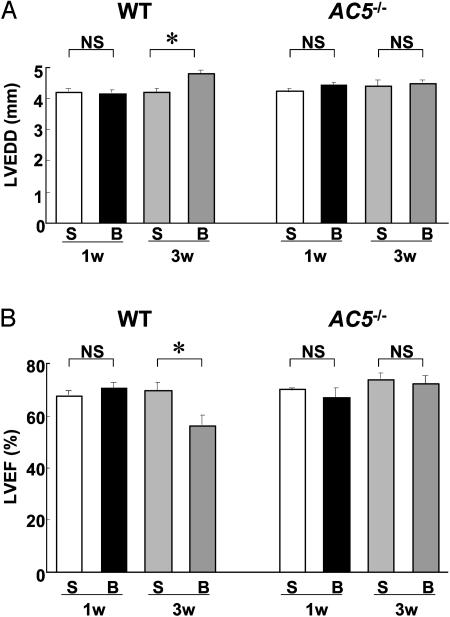
Cardiac Function Was Preserved in AC5–/– After 3 Wk of Aortic Banding. LV dimensions and cardiac function were evaluated echocardiographically. There was no difference in LVEDD and LVEF between WT and AC5–/– at baseline and at 1 wk after banding when they were compared with each other or to sham-operated animals (Fig. 2). At 3 wk after banding, however, LVEDD was significantly increased in WT whereas it remained unchanged in AC5–/– (Fig. 2 A). Similarly, LVEF fell significantly from 70 ± 2.8% to 57 ± 3.9% (P < 0.05, n = 8–11) in WT whereas it remained unchanged at 74 ± 2.2% in AC5–/– (Fig. 2B). These results suggest that cardiac function was protected after chronic pressure overload in AC5–/–. Those differences were not due to a difference in pressure gradient, which was similar at 1 wk after banding in AC5–/– (102 ± 8.2 mmHg) vs. WT (112 ± 3.1 mmHg). Heart rate was not significantly different in WT and AC5–/– under anesthesia during echocardiography but was elevated in the conscious state in AC5–/– (25).
Fig. 2.
Changes in LV function after banding in WT and AC5–/–. Echocardiographic measurements of LV function [LVEDD (A) and LVEF (B)] were performed in WT and AC5–/– after 1 and 3 wk of banding. The data (B, banding) were compared with those from sham-operated (S) controls at 1 and 3 wk. LVEDD was significantly increased (A) and LVEF was significantly decreased (B) after 3 wk of banding in WT (n = 8) but not in AC5–/– (n = 10), whereas both determinations were unchanged between in sham-operated and 1-wk-banded mice in WT (n = 6) and AC5–/– (n = 6). *, P < 0.05; NS, not significant.
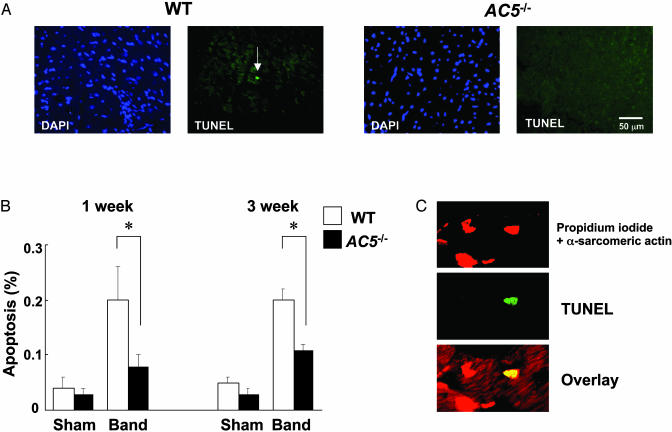
Apoptosis Was Protected in AC5–/– at 1 Wk of Banding. Before banding, there was no difference in the number of TUNEL-positive cells between the two groups, suggesting that the lack of type 5 AC did not alter the viability of cardiac myocytes at baseline. Of course, very minor differences in amounts of apoptosis could have been missed due to the accuracy of the technique, which is well suited to discern a difference such as occurs with aortic banding, but not differences in the amount of 0.05%. Aortic banding increased the number of TUNEL-positive cells in WT ≈4-fold, at both 1 and 3 wk after aortic banding (Fig. 3). The increase in apoptosis was roughly half that of WT at 3 wk and less at 1 wk after banding (Fig. 3). Analyses using confocal microscopy confirmed that apoptosis is found in cardiac myocytes (Fig. 3C) and that more apoptosis in cardiac myocytes takes place in WT than in AC5–/– in response to 1- and 3-wk aortic banding.
Fig. 3.
Comparison of TUNEL staining after banding between WT and AC5–/–. (A) DAPI (4′,6-diamidino-2-phenylindole) staining and TUNEL staining of the same fields of LV myocardium at 3 wk after aortic banding in WT and AC5–/–. A white arrow indicates a TUNEL-positive myocyte nucleus. (Bar = 50 μm.) (B) TUNEL-positive myocytes in LV myocardium were counted in WT and AC5–/– and expressed as % of myocytes. The number of TUNEL-positive myocytes was significantly smaller in AC5–/– than in WT after either 1 or 3 wk of banding (n = 6 each). *, P < 0.05. (C) An example of images from the confocal microscopic analysis showing nuclear fragmentation of a cardiac myocyte in WT banded for 1 wk. Triple staining (propidium iodide, TUNEL, and anti-α-sarcomeric actin antibody) was performed. Staining for propidium iodide and anti-α-sarcomeric actin antibody is red, and staining for TUNEL is green. In the overlay image, a nucleus stained by both TUNEL and propidium iodide is yellow.
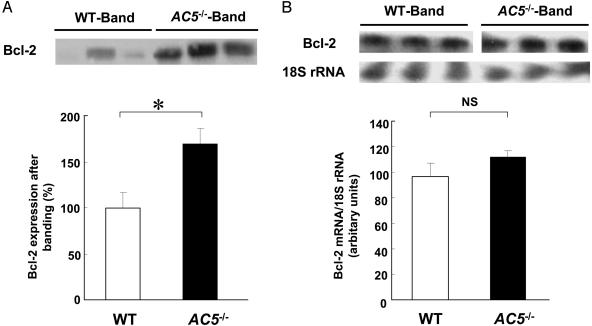
Expression of Bcl-2 Is Enhanced in AC5–/– Hearts in Response to Pressure Overload. To examine changes in the molecules that are involved in apoptosis signaling, we quantitated Bcl-2, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in WT and AC5–/– (Fig. 4). Bcl-2 expression was hardly detectable in the sham groups (data not shown). Interestingly, Bcl-2 protein expression was up-regulated after 3 wk of banding in both WT and AC5–/– although the magnitude of the increment was greater, P < 0.05, in AC5–/– (Fig. 4A). On the other hand, Bax expression was not different in the sham and banded groups (data not shown). We also examined the mRNA expression of Bcl-2. In parallel with Bcl-2 protein, mRNA of Bcl-2 was up-regulated after 3 wk of banding in both WT and AC5–/–, but the magnitude of the increment was not different between WT and AC5–/– (Fig. 4B). These results suggest that the apoptotic process is attenuated, at least in part, through the posttranscriptional regulation of Bcl-2 in AC5–/– hearts.
Fig. 4.
Western blotting and RNase protection assay of Bcl-2 after banding in WT and AC5–/–.(A) Expression of Bcl-2 after 3 wk of aortic banding was compared between WT and AC5–/–. Protein expression of Bcl-2 was determined by Western blot analysis. Although Bcl-2 was hardly detectable in the sham groups (data not shown), it was detected after banding in both WT and AC5–/–; however, expression of Bcl-2 in AC5–/– was greater than that in WT. The Bcl-2 expression level in WT after banding was taken as 100% in each experiment. *, P < 0.05, n = 9–10. (Upper) Representative immunoblots of Bcl-2 after separation by 4–20% SDS/PAGE are shown. (B) The mRNA level of Bcl-2 after 3 wk of banding was compared between WT and AC5–/–. mRNA of Bcl-2 was determined by RNase protection assays. Relative intensity of Bcl-2 to 18S rRNA is shown in the bar graph. (Upper) Representative autoradiograph of Bcl-2 and 18S rRNA is shown, n = 5. NS, not significant.
Discussion
AC5–/– mice tolerated pressure overload better than WT controls. Although the disruption of type 5 AC did not affect the development of hypertrophy, our findings demonstrated that limiting β-AR signaling at the level of type 5 AC protected the heart from decompensation potentially through decreasing the occurrence of myocardial apoptosis before cardiac dysfunction became manifest. At 1 wk after aortic banding, there were no differences in LV function between WT and AC5–/– mice. However, at 3 wk after banding, the heart dilated and LVEF was reduced in WT. In contrast, the banded AC5–/– mice were protected and maintained LV function. Because these experiments were conducted under anesthesia, myocardial depressant factors could have contributed to the decline in LV function, but, importantly, myocardial depression was not observed in AC5–/–, which received identical amounts of anesthesia.
Although acute sympathetic stimulation and activation of the cAMP-PKA pathway is a major mechanism to improve cardiac function, previous studies using transgenic models demonstrated that chronic activation of these pathways caused by the cardiac-specific overexpression of β-AR, Gsα, and PKA resulted in cardiomyopathy (8–15). Previous studies in which type 5 or type 6 AC was overexpressed do not support this position (16–22). Transgenic mice with cardiac-specific overexpression of type 6 AC did not develop cardiac dysfunction and myocardial fibrosis even in 19-mo-old animals, despite a 20-fold increase of type 6 AC protein in the heart (20). Previous studies in Gq/type 5 or 6 AC double cardiac-specific overexpression mice demonstrated that the addition of AC ameliorated Gq-mediated cardiomyopathy and improved survival in Gq mice (16, 18, 19). Furthermore, mice with overexpression of type 8 AC also presented no signs of cardiomyopathy, despite a 7-fold increase in basal AC activity and a 4-fold increase in PKA activity in the heart (21, 22). These studies, taken together, could be interpreted to indicate that increased AC in the heart is beneficial. Our results in mice with disruption of type 5 AC suggest a directionally opposite interpretation because the absence of type 5 AC did not adversely affect baseline cardiac function and actually improved the response to pressure overload.
We cannot reconcile the differences among these studies at the present time. However, it should be noted that our investigation used a knockout of type 5 AC, not an overexpression, and superimposed a pathophysiological stress, i.e., chronic pressure overload. Our experimental design is quite different from cardiomyopathy developing from gain of function mouse models. Secondly, the AC5–/– mice exhibit an unusual phenotype (25). Type 5 AC, although important in sympathetic transmission in the heart, is also a major Gi- and Ca2+-inhibitable isoform of AC (28, 29). Thus, the AC5–/– mice demonstrated attenuated responses to isoproterenol but also impaired muscarinic inhibition of sympathetic stimulation, with a corresponding alteration in vitro of inhibition of AC by Ca2+ (25). It is interesting to speculate that these latter functions of type 5 AC are not dependent on β-AR signaling, per se, but may have mediated the beneficial effects and resistance to apoptosis. However, this possibility is less likely in view of Gi's well-recognized role in protecting the heart against apoptosis (30, 31).
The data on Bcl-2 may explain the difference in apoptosis, which was enhanced more in AC5–/– than in WT with pressure overload, but Bax was not affected. We also showed that expression of Bcl-2 mRNA after aortic banding was not different between WT and AC5–/–, indicating that the difference in expression of the proteins after banding in AC5–/– and WT was likely posttranscriptional. There is considerable evidence that Bcl-2 expression is regulated by PKA and cAMP. It was reported that PKA modifies the function as well as the stability of Bcl-2 protein, in addition to the transcriptional regulation of Bcl-2 (32, 33). The activity of Bcl-2 is reduced by PKA-mediated phosphorylation in breast cancer cells (32); and, in prostatic epithelial cells, a cAMP-dependent mechanism also seems to enhance degradation of Bcl-2 possibly through phosphorylation (34). Thus, it is possible that the stability of Bcl-2 is enhanced by the reduced PKA-mediated phosphorylation of Bcl-2 in AC5–/–.
The data from the current investigation, taken together with the existing literature, suggest that the role of AC in the pathogenesis of heart failure may be isoform specific. Our findings suggest that limiting β-AR signaling at the level of a specific AC isoform, i.e., type 5 AC, did not alter the development of cardiac hypertrophy, but instead significantly protected the development of cardiac dysfunction after chronic pressure overload. Apoptosis, another consequence of pressure overload, was also protected in the AC5–/– mice. The fact that the increase in apoptosis in WT mice preceded the development of cardiac dysfunction suggests that the apoptosis may play a role in the development of heart failure by pressure overload. The protection against apoptosis and also against development of cardiac dysfunction after LV pressure overload in AC5–/– makes this molecule potentially important to study for future pharmacotherapy, where suppressing the activity of type 5 AC, and not the entire β-AR signaling pathway, may have an advantage over current β-AR blockade therapy in the treatment of heart failure.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL59729, HL61476, HL67724, HL65183, HL65182, HL69020, HL59139, AG14121, and HL33107 and by American Heart Association Grants 9940187N, 9950673N, and 0020323U.
Abbreviations: LV, left ventricular; LVEF, LV ejection fraction; LVEDD, LV end-diastolic diameter; LVW, LV weight; PKA, protein kinase A; AC, adenylyl cyclase; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; TL, tibial length; β-AR,β-adrenergic receptor.
References
- 1.Kannel, W. B., Castelli, W. P., McNamara, P. M., McKee, P. A. & Feinleib, M. (1972) N. Engl. J. Med. 287, 781–787. [DOI] [PubMed] [Google Scholar]
- 2.Morisco, C., Sadoshima, J., Trimarco, B., Arora, R., Vatner, D. E. & Vatner, S. F. (2003) Am. J. Physiol. 284, H1043–H1047. [DOI] [PubMed] [Google Scholar]
- 3.Narula, J., Pandey, P., Arbustini, E., Haider, N., Narula, N., Kolodgie, F. D., Dal Bello, B., Semigran, M. J., Bielsa-Masdeu, A., Dec, G. W., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 8144–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vatner, S. F., Vatner, D. E. & Homcy, C. J. (2000) Circ. Res. 86, 502–506. [DOI] [PubMed] [Google Scholar]
- 5.Vatner, D. E., Asai, K., Iwase, M., Ishikawa, Y., Shannon, R. P., Homcy, C. J. & Vatner, S. F. (1999) Am. J. Cardiol. 83, 80–85. [DOI] [PubMed] [Google Scholar]
- 6.Bristow, M. R., Gilbert, E. M., Abraham, W. T., Adams, K. F., Fowler, M. B., Hershberger, R. E., Kubo, S. H., Narahara, K. A., Ingersoll, H., Krueger, S., et al. (1996) Circulation 94, 2807–2816. [DOI] [PubMed] [Google Scholar]
- 7.Packer, M., Bristow, M. R., Cohn, J. N., Colucci, W. S., Fowler, M. B., Gilbert, E. M. & Shusterman, N. H. (1996) N. Engl. J. Med. 334, 1349–1355. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt, S., Hein, L., Wiesmann, F. & Lohse, M. J. (1999) Proc. Natl. Acad. Sci. USA 96, 7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisognano, J. D., Weinberger, H. D., Bohlmeyer, T. J., Pende, A., Raynolds, M. V., Sastravaha, A., Roden, R., Asano, K., Blaxall, B. C., Wu, S. C., et al. (2000) J. Mol. Cell. Cardiol. 32, 817–830. [DOI] [PubMed] [Google Scholar]
- 10.Liggett, S. B., Tepe, N. M., Lorenz, J. N., Canning, A. M., Jantz, T. D., Mitarai, S., Yatani, A. & Dorn, G. W., II (2000) Circulation 101, 1707–1714. [DOI] [PubMed] [Google Scholar]
- 11.Du, X. J., Auteliano, D. J., Dilley, R. J., Wang, B., Dart, A. M. & Woodcock, E. A. (2000) Circulation 101, 71–77. [DOI] [PubMed] [Google Scholar]
- 12.Iwase, M., Bishop, S. P., Uechi, M., Vatner, D. E., Shannon, R. P., Kudej, R. K., Wight, D. C., Wagner, T. E., Ishikawa, Y., Homcy, C. J., et al. (1996) Circ. Res. 78, 517–524. [DOI] [PubMed] [Google Scholar]
- 13.Iwase, M., Uechi, M., Vatner, D. E., Asai, K., Shannon, R. P., Kudej, R. K., Wagner, T. E., Wight, D. C., Patrick, T. A., Ishikawa, Y., et al. (1997) Am. J. Physiol. 272, H585–H589. [DOI] [PubMed] [Google Scholar]
- 14.Asai, K., Yang, G. P., Geng, Y. J., Takagi, G., Bishop, S., Ishikawa, Y., Shannon, R. P., Wagner, T. E., Vatner, D. E., Homcy, C. J., et al. (1999) J. Clin. Invest. 104, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antos, C. L., Frey, N., Marx, S. O., Reiken, S., Gaburjakova, M., Richardson, J. A., Marks, A. R. & Olson, E. N. (2001) Circ. Res. 89, 997–1004. [DOI] [PubMed] [Google Scholar]
- 16.Tepe, N. M. & Liggett, S. B. (1999) FEBS Lett. 458, 236–240. [DOI] [PubMed] [Google Scholar]
- 17.Tepe, N. M., Lorenz, J. N., Yatani, A., Dash, R., Kranias, E. G., Dorn, G. W., II, & Liggett, S. B. (1999) Biochemistry 38, 16706–16713. [DOI] [PubMed] [Google Scholar]
- 18.Roth, D. M., Gao, M. H., Lai, N. C., Drumm, J., Dalton, N., Zhou, J. Y., Zhu, J., Entrikin, D. & Hammond, H. K. (1999) Circulation 99, 3099–3102. [DOI] [PubMed] [Google Scholar]
- 19.Roth, D. M., Bayat, H., Drumm, J. D., Gao, M. H., Swaney, J. S., Ander, A. & Hammond, H. K. (2002) Circulation 105, 1989–1994. [DOI] [PubMed] [Google Scholar]
- 20.Gao, M. H., Lai, N. C., Roth, D. M., Zhou, J., Zhu, J., Anzai, T., Dalton, N. & Hammond, H. K. (1999) Circulation 99, 1618–1622. [DOI] [PubMed] [Google Scholar]
- 21.Lipskaia, L., Defer, N., Esposito, G., Hajar, I., Garel, M. C., Rockman, H. A. & Hanoune, J. (2000) Circ. Res. 86, 795–801. [DOI] [PubMed] [Google Scholar]
- 22.Georget, M., Mateo, P., Vandecasteele, V., Jurevicius, J., Lipskaia, L., Defer, N., Hanoune, J., Hoerter, J. & Fischmeister, R. (2002) FASEB J. 16, 1636–1638. [DOI] [PubMed] [Google Scholar]
- 23.Tobise, K., Ishikawa, Y., Holmer, S. R., Im, M. J., Newell, J. B., Yoshie, H., Fujita, M., Susannie, E. E. & Homcy, C. J. (1994) Circ. Res. 74, 596–603. [DOI] [PubMed] [Google Scholar]
- 24.Espinasse, I., Iourgenko, V., Defer, N., Samson, F., Hanoune, J. & Mercadier, J. J. (1995) J. Mol. Cell. Cardiol. 27, 1789–1795. [DOI] [PubMed] [Google Scholar]
- 25.Okumura, S., Kawabe, J., Yatani, A., Takagi, G., Lee, M.-C., Hong, C., Liu, J., Takagi, I., Sadoshima, J., Vatner, D. E., et al. (2003) Circ. Res., in press. [DOI] [PubMed]
- 26.Meguro, T., Hong, C., Asai, K., Takagi, G., McKinsey, T. A., Olson, E. N. & Vatner, S. F. (1999) Circ. Res. 84, 735–740. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa, Y., Sorota, S., Kiuchi, K., Shannon, R. P., Komamura, K., Katsushika, S., Vatner, D. E., Vatner, S. F. & Homcy, C. J. (1994) J. Clin. Invest. 93, 2224–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa, Y. & Homcy, C. J. (1997) Circ. Res. 80, 297–304. [DOI] [PubMed] [Google Scholar]
- 29.Defer, N., Best-Belpomme, M. & Hanoune, J. (2000) Am. J. Physiol. 279, F400–F416. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, W. Z., Zheng, M., Koch, W. J., Lefkowitz, R. J., Kobilka, B. K. & Xiao, R. P. (2001) Proc. Natl. Acad. Sci. USA 98, 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Communal, C., Colucci, W. S. & Singh, K. (2000) J. Biol. Chem. 275, 19395–19400. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava, R. K., Srivastava, A. R., Korsmeyer, S. J., Nesterova, M., Cho-Chung, Y. S. & Longo, D. L. (1998) Mol. Cell. Biol. 18, 3509–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, B. E., Mochon, E. & Boxer, L. M. (1996) Mol. Cell. Biol. 16, 5546–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Untergasser, G., Rumpold, H., Plas, E., Madersbacher, S. & Berger, P. (2001) FASEB J. 15, 673–683. [DOI] [PubMed] [Google Scholar]