Abstract
Background: The purpose of this study was to describe our experiences and analyze the benefits of video-assisted thoracoscopic surgery (VATS) combined with positron emitted tomography (PET)-computed tomography (CT) in the diagnosis of patients with early (Stage 1) sarcoidosis. Methods: From 1995 to 2006, seven patients (two males, five females), with ages ranging from 26 to 58 years, were impressed with Stage 1 sarcoidosis (mediastinal or hilar lymph nodes involvements without lung involvement) by histological examination of intrathoracic lymph nodes (LNs) and/or lung parenchyma taken from VATS biopsy. Three of them received PET or PET-CT evaluation. VATS was approached from the right and left side in one and six patients, respectively, according to the locations of their lesions. Results: All the VATS biopsied LNs or lung specimens were adequate for establishing diagnosis. Mediastinal LNs were taken from Groups 3, 4 in four, Group 7 in two, and Groups 5, 6 in one of them. Hilar LNs biopsies were performed in four cases. Lung biopsy was performed in all but two cases. All of them were expressed pathologically or radiologically as Stage 1 sarcoidosis. PET-CT revealed high emission signals over these affected LNs. These patients received oral steroid treatment or follow up only. All of them were followed up from 5 months to 11 years with satisfactory results. Conclusion: VATS biopsy is a minimally invasive, safe and effective procedure. It can be used as a diagnostic alternative of transbronchial lung biopsy (TBLB), and can harvest larger and more areas of specimens than mediastinoscopy for staging patients with sarcoidosis. PET-CT can provide us more accurate information about the characteristics and localization of these lesions before biopsy. VATS combined with PET-CT can provide more accurate and earlier diagnosis of patients with unknown intrathoracic lesions, including the sarcoidosis.
Keywords: Sarcoidosis, Video-assisted thoracoscopic surgery (VATS), Positron emitted tomography-computed tomography (PET-CT)
INTRODUCTION
Sarcoidosis, a multisystem granulomatous disease of unknown etiology, predominantly affects the lungs and intrathoracic lymph nodes (LNs). The various forms of the presentations and the lack of reliable diagnostic test of this disease make the diagnosis challenging (Newman et al., 1997; Cosabel, 2001; Geraint James and Figueroa Lebron, 1979). Tissue diagnosis is usually needed in supporting the clinical and radiologic findings, to differentiate sarcoidosis from other diseases manifested as intrathoracic LNs enlargement (Miller et al., 1995; Nishimura et al., 1993). Video-assisted thoracoscopic surgery (VATS), which was rapidly developed in the past decade, has played an important role in the diagnosis and treatment of thoracic disease (Luh and Liu, 2006; Mack et al., 1992). Computed tomography (CT) has been used as the choice of imaging diagnosis of intrathoracic lesions before the appearance of positron emission tomography (PET), which has been proven to be significantly more accurate in the distinction between benign and malignant lesions (Schrevens et al., 2004). However, false-positive on PET or PET-CT exists in patients with inflammatory granulomas (Lim and Keng, 2005), such as tuberculosis or sarcoidosis, and VATS can differentiate the malignant mass or granuloma from other benign lesions.
Patients with intrathoracic sarcoidosis can be staged radiographically (Geraint James and Figueroa Lebron, 1979): Stage 0: no radiologic abnormality; Stage 1: hilar/mediastinal lymphadenopathy alone; Stage 2: hilar/mediastinal lymphadenopathy and diffuse interstitial infiltrates; Stage 3: diffuse interstitial infiltrates without hilar/mediastinal lymphadenopathy; Stage 4: advanced chronic changes with coarse interstitial markings, honeycombing, and hilar retraction. Our experiences of using VATS and PET-CT in the diagnosis of Stage 1 sarcoidosis are described here.
PATIENTS AND METHODS
From May 1995 to January 2006, seven patients including two men and five women, aged from 26 to 58 years (averaged 44.6 years), with diagnosis of Stage 1 (hilar/mediastinal lymphadenopathy without pulmonary involvement) (Geraint James and Figueroa Lebron, 1979) after a series of evaluation including VATS biopsy, were included in this study. The criteria for the diagnosis of sarcoidosis included compatible clinical, radiological findings, as well as histologic evidence of noncaseating granuloma, and without evidence of mycobacterial infection, aerocontaminants or related medications exposure. The CT or pulmonary function test of all these patients were essentially normal or with only minimal changes. Before VATS operation, all but one patient were subjected to transbronchial lung biopsy (TBLB), which did not exclude the possibility of primary or metastatic malignancies.
VATS procedures were performed under general anesthesia, with one-lung selective ventilation contralateral to the affected side, by utilizing a double-lumen Carlens tube. Patients were placed on lateral decubitus position, as we do in case of posterolateral thoracotomy. The first port used for thoracoscope insertion, was usually positioned in the 5th to 7th intercostal space on the mid- or posterior axillary line. The other one to two accesses were placed according to the position of the lesions. The whole hemithorax can be visualized by this approach. We used routine VATS biopsies for patients with abnormal Stations 5, 6 (sub- and para-aortic), 7 (subcarinal) and 8, 9 (para-esophageal and inferior pulmonary ligament) lymph nodes, as well as Stations 2 to 4 (pre- and para-tracheal) lymph nodes with suspected lung lesions requiring additional biopsy. The LNs from right para-, pre-tracheal (Groups 2, 3, 4), subcarinal (Group 7) or right hilar area were approached from the right side. However, LNs from sub-, para-aortic (Groups 5, 6) or left hilar area would approach from the left side. The histological specimens from the frozen and paraffin sections were examined. Several times of biopsies at the same or different areas of LNs were usually needed for confirming diagnosis. If non-caseating granulomas were impressed histologically from frozen section, lung biopsies with blinded pattern or based on the CT finding would be performed in some of our patients to detect possible pulmonary involvement of sarcoidosis.
The CT simulation followed by [18F] FDG-PET (PET with the glucose analogue tracer 2-[fluorine-18] fluoro-2-deoxy-D glucose) was performed in three of our patients, using a commercial software package based on surface matching technique. An irregular region of interest (ROI) was drawn on [18F] FDG-PET images, which were automatically and digitally put on the co-registered CT images. Detailed description of the image acquisition, fusion, analysis and contour delineation have been described in (Messa et al., 2005).
RESULTS
Before the diagnosis, all of these seven patients were found with non-specific symptoms, such as fever, fatigue, body weight loss, arthralgia, non-productive cough or minimal exertional dyspnea. None of them was found with prominent pulmonary or extra-pulmonary organ involvements, such as skin nodules, hepatic granulomas, uveitis, myocarditis, or neuropathies. Abnormalities of the laboratory data revealed mild leucopenia in two (28.6%), and hypercalcemia in one (14.3%) of these seven patients. Kveim reaction was tested pre- or post-VATS biopsy in two of them but the results were negative. Pulmonary function tests of these patients showed normal or only mild restrictive ventilatory impairments. Chest X-ray and high resolution computed tomography (HRCT) abnormalities were found preoperatively in all of these patients, with the presentation of mediastinal and/or hilar lymphadenopathy, which were distributed bilaterally in five, predominantly on the left side in one, and on the right side in one of them. None of them was found with pulmonary infiltration on HRCT imaging. PET-CT scans were performed in three of them, revealing increased signals over the affected LNs (Fig.1).
Fig. 1.
The PET-CT patterns for patient with Stage 1 sarcoidosis, in which strong emission signals were noted in the mediastinal and hilar lymph nodes
LNs biopsy through VATS was approached from the right side in six, and from the left side in one of them. All the VATS biopsied LNs or lung specimens were adequate for establishing diagnosis. Mediastinal LNs were taken from Groups 3, 4 in four, Group 7 in two, and Groups 5, 6 in one of them (Fig.2). Hilar LNs biopsies were performed in four cases. Lung biopsy was performed in all but two cases. All of their specimens taken for pathological examinations revealed non-caseating granulomas (Fig.3), and sarcoidosis was impressed after exclusion of other possible diagnosis. All of these patients underwent tuberculous polymerase chain reaction (TB PCR) to exclude the possibility of tuberculosis.
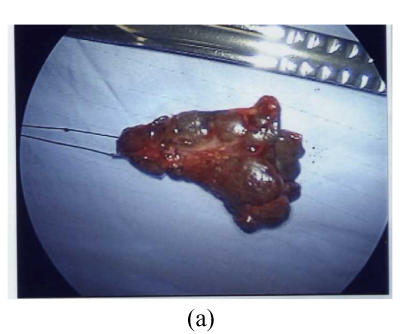
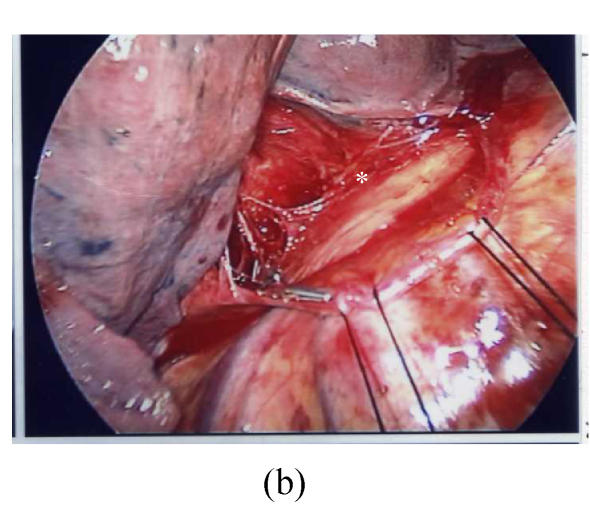
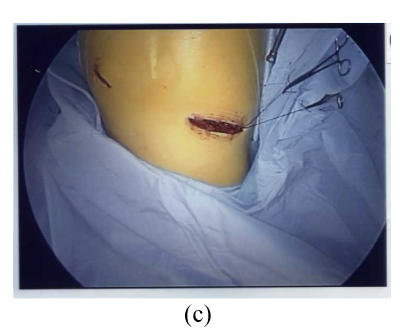
Fig. 2.
Thoracoscopic findings in patients with Stage 1 sarcoidosis. In this patient the Group 7 (subcarinal) lymph node was the most prominent affected site. (a) The resected Group 7 lymph node; (b) Thoracoscopic findings after removal of the lymph node, the space was shown in * area; (c) The wound after VATS procedure
Fig. 3.
Histopathological findings of these patients revealing non-caseating granuloma formation (H & E stain, 250×)
These patients received oral and inhaled steroid therapy (n=5) or did not receive special treatments (n=2). They were followed from 5 months to 11 years with disease remission or stable condition.
DISCUSSIONS AND CONCLUSION
Sarcoidosis was first described as a dermatologic disorder in 1877 (Hutchinson, 1877). The systemic nature and associated pulmonary involvement were uncovered nearly 40 years later (Kuznitsky and Bittorf, 1915). It is usually present in adults younger than 40 years, is slightly more predominant in women than in men, and is most prevalent in Swedes and US blacks (Cosabel, 2001). The causes of sarcoidosis are still unclear, despite many possible factors, such as genetic inheritance, infectious transmission, and exposure to environmental agents, and have never been reported (Cosabel, 2001; ATS, 1999).
Clinical presentation of sarcoidosis depends on ethnicity, extent of organ involvement, duration of illness, and activity of the disease process (Geraint James and Figueroa Lebron, 1979; Johns et al., 1989). Lungs are the most commonly involved organs, followed by skin, eyes, nervous system, heart, kidneys, and musculoskeletal systems (ATS, 1999). Lofgren (1953)’s syndrome, a specific type of sarcoidosis, which is characterized by the abrupt onset of mediastinal and hilar lymphadenopathy, fever, and erythema nodosum, is frequently considered as an indication for mediastinal or hilar LNs biopsy because it cannot be distinguished from other intrathoracic benign or malignant lesions, such as tuberculosis, lymphomas or metastatic carcinomas (Lofgren, 1953; Reich, 2003).
The diagnosis of sarcoidosis remains non-specific, based on a typical clinical presentation, compatible imaging, physiologic studies, a pathological examination revealing non-caseating granulomas, and the absence of a fungal or mycobacterial infection (Martin et al., 2004). Certain biologic markers, such as the CD4 to CD8 ratio being greater than 3.5 in bronchoalveolar lavage fluid, panda and lambda patterns on gallium scan, or an angiotensin-converting enzyme (ACE) level double the normal value may help for diagnosis but not specific for sarcoidosis (Cosabel, 2001; ATS, 1999). New approaches to the diagnosis of sarcoidosis are still evolving, such as biopsy or imaging techniques, as well as the diagnosis techniques based on related genomic or proteomic technology.
Tissues to diagnose pulmonary or mediastinal sarcoidosis can be yielded through various biopsy techniques before. A bronchoscopy with TBLB is the preferred diagnostic test (Gilman and Wang, 1980). Mediastinoscopic (McNeill and Chamberlain, 1966) or VATS approaches are usually reserved for cases when other measures cannot yield the diagnosis. There have been still very limited reports about the application of VATS in the diagnosis of sarcoidosis in (Massone et al., 2003; Ohmichi, 2002; Nakayama et al., 2000; Gould et al., 2003). In TB high prevalence area, patients with hilar or mediastinal lymphadenopathy should be differentiated pathologically between sarcoidosis and tubeculosis (Nakayama et al., 2000). VATS biopsy is a minimally invasive, safe and effective procedure, which can harvest larger and more areas of specimens than the traditional TBLB, to distinguish different diseases. VATS, compared with mediastinoscopy, can provide additional lung biopsy while indicated for staging patients with sarcoidosis. The choice of site for VATS LNs/lung biopsy has been suggested in the present study and can be determined by pre-operative imaging studies. Para-tracheal or sub-carinal LNs are better approach through the right side of chest, and para-aortic or sub-aortic LNs are better approach from the left side.
[18F] FDG-PET has been proven more accurate than CT for the differentiation between intrathoracic benign and malignant lesions (Sharma et al., 2004; Brigid et al., 1997). However, certain physiologic or benign pathologic conditions would be mistaken for cancer on FDG-PET images. Various physiologic FDG uptakes could be found in the digestive tract, thyroid gland, skeletal muscle, myocardium, bone marrow and genitourinary tract (Shreve et al., 1999). Certain benign pathologic FDG uptakes could also be found, such as healing bone, lymph nodes, joints, and sites of infection (Rizzo et al., 2001). Thus, FDG uptake in lymph nodes is not specific for a malignant neoplasm. Active granulomatous diseases such as tuberculosis and sarcoidosis cause high FDG uptake in involved lymph nodes (Beyer et al., 2000). Since combined PET-CT can make accurate localization of “hot spots” in CT scan, it can provide us a relatively good guide for tumor or lymph node biopsy for intrathoracic lesions (Beyer et al., 2000; Paramothayan and Jones, 2002). A study comparing the diagnostic values of [18F] FDG-PET and 67Ga scintigraphy, noted that [18F] FDG-PET can detect pulmonary lesions to a similar degree as 67Ga scintigraphy. However, [18F] FDG-PET appears to be more accurate and contributes to a better evaluation of extrapulmonary involvement in sarcoidosis patients (Nishiyama et al., 2006). In the present study, PET-CT was used for determination of best possible biopsy sites, which can effectively decrease the biopsy sites (either lung or LNs) to obtain more accurate information.
The values of our approaches to diagnose Stage 1 sarcoidosis were as follows. Firstly, the PET-CT scan can provide more precise localization of the “dysfunctional area”, which can be as a better guide for biopsy than the conventional CT scan. Secondly, since other clinical manifestations, including the pulmonary or extra-pulmonary areas, are unremarkable and non-specific, for patients at the Stage 1 sarcoidosis, the biopsy through VATS becomes a good alternative tool for accurate tissue diagnosis, which is superior to mediastinoscopy or bronchoscopy because VATS can check the pathology of mediastinal LN and pulmonary hilar LN, as well as pulmonary parenchyma in one procedure. The yields of VATS, in our experiences, were usually superior to those of transbronchial or mediascopic biopsies. The VATS is as safe a procedure as the other two approaches. In addition, earlier tissue diagnosis of patients with mediastinal lymphadenopathy can exclude other possible diseases, such as the lymphoma, metastatic carcinomas, or other granulomatous diseases. This can provide us a better guideline and earlier timing for the subsequent therapies.
In conclusion, VATS biopsy is a minimally invasive, safe and effective procedure. It can be used as a diagnostic alternative of TBLB when the later cannot get adequate specimens for accurate diagnosis. VATS can also harvest larger and more areas of specimens (lungs and LNs from both mediastinum and hilum) than the mediastinoscopy for staging patients with sarcoidosis. PET-CT can provide us more accurate information about the characteristics and localization of these lesions before VATS biopsy.
References
- 1.ATS (American Thoracic Society) Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 3.Brigid GA, Flanagan FL, Dehdashti F. Whole-body positron emission tomography: normal variations, pitfalls, and technical considerations. AJR. 1997;169:1675–1680. doi: 10.2214/ajr.169.6.9393189. [DOI] [PubMed] [Google Scholar]
- 4.Cosabel U. Sarcoidosis: clinical update. Eur Respir J Suppl. 2001;18(32):56s–68s. [PubMed] [Google Scholar]
- 5.Geraint James D, Figueroa Lebron RE. Update on sarcoidosis. Bol Asoc Med P R. 1979;71:325–335. [PubMed] [Google Scholar]
- 6.Gilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. Ann Rev Respir Dis. 1980;122:721–724. doi: 10.1164/arrd.1980.122.5.721. [DOI] [PubMed] [Google Scholar]
- 7.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, Chan JK, Owens DK. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson J. Anomalous Disease of the Skin of the Fingers. Papillary Psoriasis. In: Hutchinson J, editor. Illustrations of Clinical Surgery. London: J and A Churchill; 1877. [Google Scholar]
- 9.Johns CJ, Scott PP, Schonfeld SA. Sarcoidosis. Annu Rev Med. 1989;40(1):353–371. doi: 10.1146/annurev.me.40.020189.002033. [DOI] [PubMed] [Google Scholar]
- 10.Kuznitsky E, Bittorf A. Boecksches sarkoid mit beteligung innerer organe. Munch Med Wochenschr. 1915;62:1349–1352. [Google Scholar]
- 11.Lim JWM, Keng GHW. False positive F-18 fluorodeoxyglucose combined PET/CT scans from suture granuloma and chronic inflammation: report of two cases and review of literature. Ann Acad Med Singapore. 2005;34:457–460. [PubMed] [Google Scholar]
- 12.Lofgren S. Primary pulmonary sarcoidosis. Acta Med Scand. 1953;145:424–431. [PubMed] [Google Scholar]
- 13.Luh SP, Liu HP. Video-assisted thoracic surgery—the past, present status and the future. J Zhejiang Univ Sci B. 2006;7(2):118–128. doi: 10.1631/jzus.2006.B0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack MJ, Aronoff R, Acuff T. The present role of thoracoscopy in the diagnosis and treatment of diseases of the chest. Ann Thorac Surg. 1992;54:405–409. doi: 10.1016/0003-4975(92)90428-7. [DOI] [PubMed] [Google Scholar]
- 15.Martin WJ, Iannuzzi MD, Gail DB, Peavy HH. Future directions in sarcoidosis research. Am J Respir Crit Care Med. 2004;170(5):567–571. doi: 10.1164/rccm.200308-1073WS. [DOI] [PubMed] [Google Scholar]
- 16.Massone PPB, Lequaglie C, Magnani B, Ferro F, Ctaldo I. The real impact and usefulness of video-assisted thoracoscopic surgery in the diagnosis and therapy of clinical lymphadenopathies of the mediastinum. Ann Surg Oncol. 2003;10(10):1197–1202. doi: 10.1245/ASO.2003.03.538. [DOI] [PubMed] [Google Scholar]
- 17.McNeill T, Chamberlain J. Diagnostic mediastinotomy. Ann Thorac Surg. 1966;2:532–539. doi: 10.1016/s0003-4975(10)66614-3. [DOI] [PubMed] [Google Scholar]
- 18.Messa C, Ceresoli GL, Rizzo G, Artioli D, Cattaneo M, Castellone P, Gregorc V, Picchio M, Landoni C, Fazio F. Feasibility of [18F] FDG-PET and coregistered CT on clinical target volume definition of advanced non-small cell lung cancer. Q J Nucl Med Mol Imaging. 2005;49:259–266. [PubMed] [Google Scholar]
- 19.Miller BH, Rosado-de-Christenson ML, McAdams HP, Fishback NF. Thoracic sarcoidosis: radiologic-pathologic correlation. Radiographics. 1995;15(2):421–437. doi: 10.1148/radiographics.15.2.7761646. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T, Hachisuka H, Furuichi S, Akusawa H, Omori C, Kayama Y, Nomura Y, Omori K, Horie T. Sarcoidosis differentially diagnosed from mediastinal tumor by thoracoscopic biopsy. Nihon Kokyuki Gakkai Zasshi. 2000;38:131–135. [PubMed] [Google Scholar]
- 21.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336(17):1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura K, Itoh H, Kitaichi M, Nagai S, Izumi T. Pulmonary sarcoidosis: correlation of CT and histopathologic findings. Radiology. 1993;189:105–109. doi: 10.1148/radiology.189.1.8372178. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, Ohkawa M. Comparative evaluation of 18F-FDG PET and 67 Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–1576. [PubMed] [Google Scholar]
- 24.Ohmichi M. Histologic diagnosis of sarcoidosis. Nippon Rinsho. 2002;60:1759–1765. [PubMed] [Google Scholar]
- 25.Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systemic review. JAMA. 2002;287(10):1301–1307. doi: 10.1001/jama.287.10.1301. [DOI] [PubMed] [Google Scholar]
- 26.Reich JM. What is sarcoidosis. Chest. 2003;124(1):367–371. doi: 10.1378/chest.124.1.367. [DOI] [PubMed] [Google Scholar]
- 27.Rizzo G, Cattaneo M, Castiglioni I, et al. Proceedings of the 23rd Annual International Conference of the IEEE. Istanbul, Turkey: 2001. Integration of CT/PET Images for the Optimization of Radiotherapy Planning. Engineering in Medicine and Biology Society. [Google Scholar]
- 28.Schrevens L, Lorent N, Dooms C, Vansteenkiste J. The role of PET scan in diagnosis, staging, and management of non-small-cell lung cancer. Oncologist. 2004;9(6):633–643. doi: 10.1634/theoncologist.9-6-633. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Fidias P, Hayman LA, Loomis SL, Taber KH, Aquino SL. Patterns of lymphadenopathy in thoracic malignancies. Radiographics. 2004;24(2):419–434. doi: 10.1148/rg.242035075. [DOI] [PubMed] [Google Scholar]
- 30.Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19(1):61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]