Abstract
In order to investigate the influencing factors of 5-hydroxymethyl-2-furaldehyde (5-HMF) content in Schisandra, confirm the theory of 5-HMF deriving mainly from Schisandra processing course, and give some suggestions about the Schisandra processing method, the 5-HMF contents in decoctions of Schisandra under different heating temperature, decocting time, soaking time, processing methods and treatment with different solvents before decocting the Schisandra were measured by RP-HPLC method. The results showed that there is great difference of 5-HMF level in decoctions from differently processed Schisandra and unprocessed Schisandra; decocting time of 60 min has some effects on 5-HMF level in decoctions and there is certain quantity 5-HMF in processed Schisandra itself and very little 5-HMF in unprocessed Schisandra. Heating time, heating temperature and treating solvents all have effect on 5-HMF level in decoction of Schisandra. 5-HMF in Schisandra was mainly from processing course. Both long heating time and high heating temperature can increase 5-HMF level in Schisandra. The production of 5-HMF in Schisandra may have some relationships with some polar components, which can dissolve in water, ethanol and acetone, especially in ethanol. To control processing temperature, processing time and treatment with some solvent is very important for controlling 5-HMF level in Schisandra.
Keywords: Schisandra process, Heating temperature, Heating time, 5-Hydroxymethyl-2-furaldehyde (5-HMF)
INTRODUCTION
Normally, 5-hydroxymethyl-2-furaldehyde (5-HMF) is considered an irritant to eyes, upper respiratory tract, skin and mucous membranes and damages striated muscles and viscera by combining to protein and thus causing the accumulation of poisons in the body (Chi et al., 1998; Pamplona et al., 1995), and may also lead to mutagenicity and carcinogenicity (Khan et al., 1995; Janzowski et al., 2000). It often takes place when fructose or glucose solutions of high concentrations encounter high temperature or acid environment (Miyazawa and Funazukuri, 2006). 5-HMF has been identified in honey (Nozal et al., 2001; Lu et al., 2006), fruit juice (Burdurlu and Karadeniz, 2003), raisins (Palma and Taylor, 2001), beer (Castellari et al., 2001), oak wood (García-Romero et al., 1998), milk (Morales and Jimenez, 1999) and instant coffee (Charlton et al., 2002). A sequence of nonenzymatic browning reactions (the so-called Maillard reaction) are initiated during heat treatment of foods containing reducing sugars and amino acids. 5-HMF is a common intermediate product in the Maillard reaction (Burdurlu and Karadeniz, 2003). Human are potentially exposed to 5-HMF through pharmceutical preparations, cigarette smoke, and the consumption of a number of commonly available beverages and foods. The content of 5-HMF in the honey, beer and glucose injection has been limited strictly (Lo Coco et al., 1995; Li et al., 2004). But in recent years, there are more and more reports about 5-HMF found in TCM (traditional Chinese medicine) and it has a positively effect to human being (Dai et al., 2001; Hou et al., 2005; Miyazawa et al., 2003; Sharma et al., 2004; Xu et al., 2004). It was reported that 5-HMF in the decoction of Shengmaiyin and believed that 5-HMF was derived from the combination of Schisandra and Ophipogon and has contributed to the cure of cardiovascular disease (Zhu et al., 1998a). Similar studies have been performed on 5-HMF origin and it was reported that 5-HMF is mainly derived from Schisandra chinensis only and that processing method plays an important role in 5-HMF production of Schisandra (Li and Lu, 2005).
According to TCM theory, there is no clear difference between poison and medicine. Some strongly effective TCMs are medicine at low dose and poison in large amount. Maybe 5-HMF in TCM plays the same role as strongly effective TCM plays. It is very important to control 5-HMF level in view of the activity and side effects of the herbs which can produce 5-HMF in the processing course. In order to control the quality of Schisandra to a level of a high curative effect with low side effects, optimize the process technique, and confirm the theory of 5-HMF deriving mainly from Schisandra processing course, the origin and influencing factors on 5-HMF level in Schisandra were investigated in this paper by measuring the 5-HMF contents under different heating temperature, decocting time, soaking time, processing methods and extracting with different solvents before decocting the Schisandra. The result can provide some suggestions about the Schisandra processing method and somehow influence the knowledge of TCM, moreover, it can give us some inspirations about the 5-HMF origin of Schisandra.
MATERIALS AND METHODS
Materials and reagents
Unprocessed Schisandra is the dry ripe fruit of Schisandra chinensis (Turcz.) Baill. Medicinal Schisandra is made from Schisandra chinensis (Turcz.) Baill and Schisandra sphenanthera Rehd. Et Wils. by different process. All crude herbal medicine and medicinal medicine were authenticated by Professor Kongrong Chen from the College of Zhejiang Traditional Chinese Medicine and all are measured up to pharmacopoeia (Ch.P.C., 2005).
HPLC grade acetonitrile (Merck, Germany), ultra-pure water (milli-Q), 5-HMF (Sigma, USA), 0.45 µm micro-filter (nylon), ethanol (CP), petroleum ether (60~90 °C), acetone (AR), deionized water.
Instruments
Agilent HP1100 HPLC system equipped with a G1322A solvent degasser, a G1354A quaternary gradient solvent pump, a G1313A multiple autosampler, a G1316A thermostatted column compartment, a G1314A UV-Vis detector, a Diamonsil C18 column (250 mm×4.6 mm, 5 μm; Dikma) with an ODS guard column (4 mm×3 mm i.d., 5 μm; Phenomenex) and Agilent HP1100 chromatography workstation was used for analyses; TC-15 canular thermostated container (Zhejiang Xinhua Medical Instrument Factory) for decocting TCM, analytical balance (0.1 mg, AL204, Mettler Toledo GmbH) for weighing standards and TCM.
Analytical methods
5-HMF of standards and samples were all analyzed by RP-HPLC (Li and Lu, 2005). The precision, stability, accuracy and recoveries of the samples and their relative standard deviations were measured to observe the HPLC performance and system suitability of the method.
Preparation of calibration curve
Stock solution of 5-HMF was prepared by dissolving 13.62 mg 5-HMF in a 50 ml volumetric flask with ultra-pure water and stored at 4 °C. Seven concentrations of 5-HMF standard solutions (2.724×10−2, 5.448×10−2, 8.172×10−2, 1.0896×10−1, 1.362×10−1, 1.6344×10−1, 2.724×10−1 mg/ml) were prepared by appropriately diluting the stock solution with mobile phase and used to evaluate the linearity by HPLC. The calibration curve was established by plotting the peak areas against the concentrations of 5-HMF standard solutions.
Sample preparation
Processed Schisandra samples of different origins and weights (6.25, 12.5, 18.75, 25, 50 g) were soaked in 250 ml deionized water for 2 h and weighed, then decocted for 1 h at a moderate boiling temperature and reweighed. Deionized water was added to reach the required weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm micro-filter for HPLC analysis.
Samples were soaked for different times: 12.5 g of Schisandra of different origins was soaked in 250 ml deionized water for 1, 2, 4, 6, 8, 10, 12 h and then the lixiviums were filtered; the filtrate was passed through a 0.45 μm micro-filter for HPLC analysis.
Decocting time of the samples was different: 12.5 g of Schisandra of different origins was soaked in 250 ml deionized water for 2 h and weighed, then decocted for 10, 20, 40, 60, 80, 100, 120 and 140 min at moderate boiling temperature and reweighed with deionized water to make up the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm micro-filter for HPLC analysis.
Decocting temperatures were as follows: 12.5 g unprocessed Schisandra samples were soaked in 250 ml deionized water for 2 h and weighed, then decocted for 1, 2, 4, 6, 8, 10, 12 h at 20, 40, 60, 80 and 100 °C and reweighed with deionized water added to make up to the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm micro-filter for HPLC analysis.
Samples were decocted after extraction in different solvents: 50 g unprocessed Schisandra was soaked into 1000 ml of different solvents (ethanol, petroleum ether, acetone and deionized water) respectively for 12 h, filtered and the residue was dried at 60 °C, then 12.5 g dried residue was soaked in 250 ml deionized water for 2 h and weighed, then decocted for 4 h at moderate boiling temperature and reweighed with deionized water added to make up the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm micro-filter for HPLC analysis.
RESULTS AND DISCUSSIONS
HPLC performance and system suitability of the method
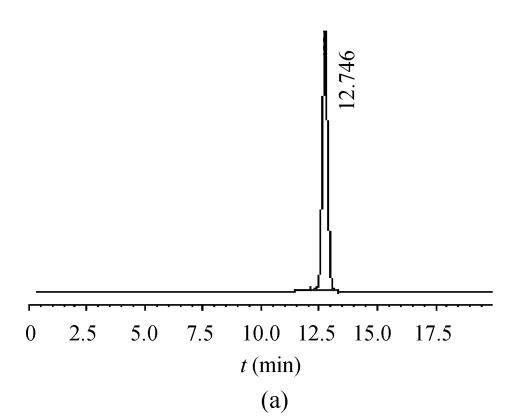
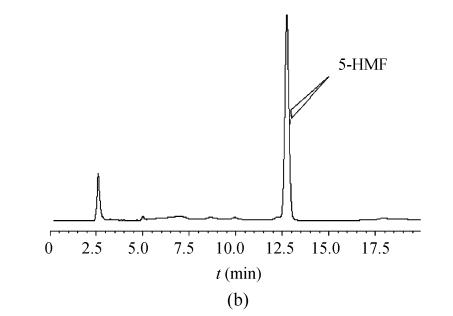
Effective separation, acceptable sensitivity, and symmetric peak shapes were achieved in a short analytical period. The retention time of 5-HMF was about 12.5 min, and no interferences were observed. The number of theoretical plates of column (N) and the tailing factor (T) for analyzing 5-HMF were about 14 300 and 0.96 respectively. The average extraction recovery of 5-HMF in decoction was 98.89%. The relative standard deviations of precision, stability, repeatability and recoveries of 5-HMF in decoction were 0.71%, 1.23%, 1.58% and 2.78% respectively. The extraction recovery data of 5-HMF is presented in Table 1. Representative chromatograms are shown in Fig.1.
Table 1.
Extraction recovery data of 5-HMF
| Sample | Found (mg) | Added (mg) | Extraction recovery (%) | Average (%) | RSD (%) |
| 1 | 13.46 | 13.62 | 98.83 | 98.89 | 2.78 |
| 2 | 13.04 | 13.62 | 95.77 | ||
| 3 | 13.64 | 13.62 | 100.12 | ||
| 4 | 13.30 | 13.62 | 97.66 | ||
| 5 | 13.25 | 13.62 | 97.31 | ||
| 6 | 14.12 | 13.62 | 103.65 |
Fig. 1.
Chromatograms of standard and sample. (a) 5-HMF standard; (b) Schisandra sample decoction
Linearity
The peak area (Y) and concentration (X) of 5-HMF standard solutions were subjected to regression analysis to calculate the calibration equation and correlation coefficient. The calibration equation was Y=7376.8X+6.8016, R 2=0.9999. The results showed excellent correlation between the peak area and the concentration of 5-HMF at the concentrations of 0.02724 to 0.2724 mg/ml.
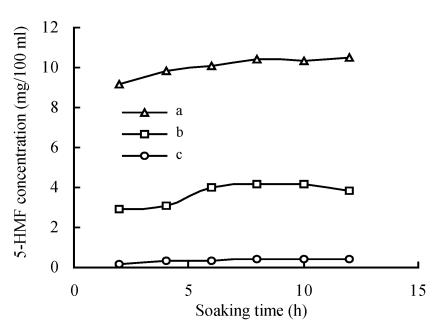
Influence of soaking time on the 5-HMF level
There was no difference between the 5-HMF level in lixivium of the Schisandra after soaking for 2 h and 12 h, but a great difference between 5-HMF levels in lixiviums of different medicinal Schisandra and unprocessed Schisandra preparations. The quantity of 5-HMF in lixivium of medicinal Schisandra was much more than that of unprocessed Schisandra (Fig.2). The results showed that medicinal Schisandra contained a certain amount of 5-HMF and that unprocessed Schisandra contained less 5-HMF. 5-HMF in processed Schisandra decoction was from the processed Schisandra itself but not from decocting course. Different processing procedures may result in different 5-HMF level.
Fig. 2.
Influence of soaking time on the 5-HMF level in Schisandra
a: Medicinal Schisandra (made by factory A); b: Medicinal Schisandra (made by factory B); c: Crude Schisandra (unprocessed)
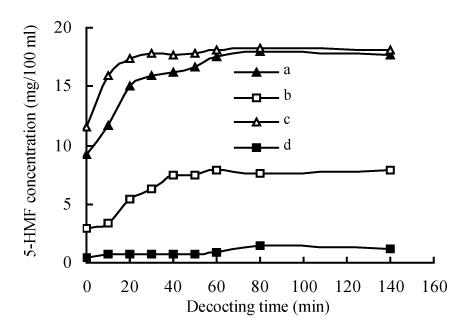
Influence of decocting time on the 5-HMF level
The 5-HMF level of the decoctions of processed Schisandra or pulverized Schisandra was evidently affected by decocting time and has positive relationship with decocting time of less than 60 min (Fig.3). Six minutes later, the level of 5-HMF in Schisandra decoctions did not increase further, probably because the 5-HMF content in the processed Schisandra was limited. After decoction for a certain time, the 5-HMF content in decoctions reaches its maximum. The result was consistent with the report that 5-HMF is not present in decoctions after three extractions (Zhu et al., 1998b). 5-HMF level in pulverized Schisandra was no more than that in unpulverized Schisandra but its solubility time has advanced. If 5-HMF is considered to be representative of active components, the theory that Schisandra has to be pulverized when used as medicine was reasonable because the pulverization of Schisandra can decrease the decocting time and protect the thermo-sensitive ingredients from being destroyed.
Fig. 3.
Effect of decocting time on the level of 5-HMF in decoctions
a: Medicinal Schisandra (made by factory A); b: Medicinal Schisandra (made by factory B); c: Medicinal Schisandra (made by factory A, comminuted); d: Crude Schisandra (unprocessed)
Influence of Schisandra origin on the 5-HMF level
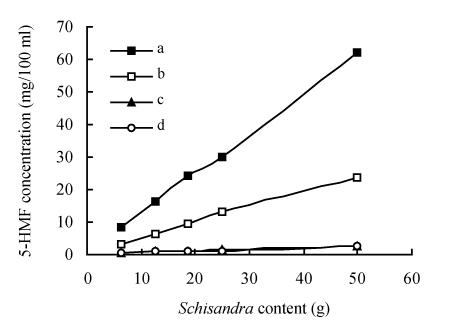
The 5-HMF level of the decoctions showed evident differences between differently processed Schisandra group decoctions and the unprocessed Schisandra group decoctions (Fig.4). There was far less 5-HMF in unprocessed Schisandra decoctions than in processed Schisandra decoctions and 5-HMF levels were proportionate to the Schisandra content. There were also great differences in the 5-HMF levels among the three kinds of medicinal Schisandra decoctions. The results showed that the processing methods have great effect on the production of 5-HMF and that the changing of 5-HMF levels in the decoctions was mainly controlled by Schisandra processing methods. The results were confirmed in the report that 5-HMF appeared in the course of Ephedra Stapf processing (Xu et al., 2004). Maybe the factors of processing time, processing temperature and herb species probably all affect 5-HMF levels in medicinal Schisandra during the course of the processing.
Fig. 4.
Influence of Schisandra origin on the production of 5-HMF
a: Medicinal Schisandra (made by factory A); b: Medicinal Schisandra (made by factory B); c: Medicinal Schisandra (made by factory C); d: Unprocessed Schisandra
Influence of decocting temperature on the 5-HMF level
The effect of temperature on 5-HMF level was significant when crude Schisandra was heated for 4 h at 100 °C. There was no difference in 5-HMF levels when the unprocessed Schisandra was decocted below 80 °C or was heated to below 100 °C within 2 h (Table 2). The results showed that high temperature and long heating time may affect 5-HMF levels evidently in the course of processing Schisandra. 5-HMF in the decoctions or lixiviums under 80 °C or within 2 h resulted in 5-HMF in Schisandra diffusing to water, and 5-HMF in the decotions was the result of chemical reaction in Schisandra when the temperature was up to 100 °C or heating time was long. If 5-HMF has a positive active function at low doses and a poisonous function at high doses according to TCM theory, it may be reasonable to steam the crude Schisandra for 4 h before using it as medicinal Schisandra.
Table 2.
Influence of decocting temperature on the 5-HMF level in crude Schisandra decoction
| Heating time (h) | 5-HMF concentration (mg/100 ml) |
||||
| 25 °C | 40 °C | 60 °C | 80 °C | 100 °C | |
| 1 | 0.08 | 0.11 | 0.24 | 0.18 | 0.13 |
| 2 | 0.18 | 0.21 | 0.32 | 0.35 | 0.34 |
| 4 | 0.33 | 0.40 | 0.33 | 0.56 | 0.72 |
| 6 | 0.33 | 0.41 | 0.35 | 0.63 | 1.45 |
| 8 | 0.45 | 0.43 | 0.46 | 0.71 | 2.19 |
| 10 | 0.43 | 0.52 | 0.45 | 0.90 | 2.68 |
| 12 | 0.45 | 0.46 | 0.47 | 0.98 | 3.21 |
Influence of different extracts on the 5-HMF level
There are great differences in 5-HMF levels in the decoction of the unprocessed Schisandra residue extracted by different solvents. 5-HMF levels in decoctions of the unprocessed Schisandra residue treated by petroleum ether were much higher than that treated by acetone, ethanol or water. 5-HMF levels in decoctions of Schisandra residues treated by acetone, ethanol or water were lower than that in decoctions of untreated Schisandra (Table 3). The results showed that 5-HMF may derive from some polar components present in Schisandra. Since there is a report about that tannin in fruit may trigger browning reaction and that browning reaction has relation with 5-HMF (Roig et al., 1999). It was also reported that 5-HMF level in immature fruit juice is higher than in ripe fruit juice and that the tannin content in immature fruit is higher than that in ripe fruit (Poll, 1985). Whether there is relationship between 5-HMF and tannin or not requires further investigation.
Table 3.
Influence of treating solvent on the 5-HMF level
| Treating solvent | 5-HMF concentration (mg/100 ml) |
| Petroleum ether | 8.06 |
| Ethanol | 0.11 |
| Acetone | 0.73 |
| Water | 0.91 |
| Without extract | 1.53 |
CONCLUSION
Processed Schisandra has much more 5-HMF content than unprocessed Schisandra. 5-HMF in Schisandra decoctions results mainly from the method of processing and was not from decocting course. Both long heating time and high heating temperature can increase 5-HMF level in Schisandra. The theory that Schisandra has to be pulverized when being used as medicine was reasonable because pulverizing Schisandra can decrease the decocting time and protect the thermo-sensitive ingredients from being destroyed. 5-HMF may derive from some polar components which are soluble in water, ethanol and acetone, especially ethanol. To control processing temperature and processing time or pretreatment by some solvents is very important if want to control 5-HMF level in Schisandra. Whether 5-HMF has relation with tannin has not been determined.
Acknowledgments
We thank Prof. Kongrong Chen for his help in herb authentication. The authors are grateful to Prof. Sarah Radloff and Prof. Randall Hepburn for editing the article in English.
Footnotes
Project supported by the Ministry of Science and Technology of China (No. 2004CCA05500), the National Natural Science Foundation of China (No. 20476089) and the Natural Science Foundation of Zhejiang Province, China (No. Y405157)
References
- 1.Burdurlu HS, Karadeniz F. Effect of storage on nonenzymatic browning of apple juice concentrates. Food Chem. 2003;80(1):91–97. doi: 10.1016/S0308-8146(02)00245-5. [DOI] [Google Scholar]
- 2.Castellari M, Sartini E, Spinabelli U, Riponi C, Galassi S. Determination of carboxylic acids, carbohydrates, glycerol, ethanol and 5-HMF in beer by high-performance liquid chromatography and UV-refractive index double detection. J Chromatogr Sci. 2001;39(6):235–238. doi: 10.1093/chromsci/39.6.235. [DOI] [PubMed] [Google Scholar]
- 3.Charlton AJ, Farrington WHH, Brereton P. Application of 1H NMR and multivariate statistics for screening complex mixtures: quality control and authenticity of instant coffee. J Agric Food Chem. 2002;50(11):3098–3103. doi: 10.1021/jf011539z. [DOI] [PubMed] [Google Scholar]
- 4.Chi W, Zhang CB, Cao YH, Guo LY. Investigation of the restriction on the formation of 5-HMF. Spec J Pharm People’s Mil Surg. 1998;14(2):101–104. (in Chinese) [Google Scholar]
- 5.Ch.P.C. (China Pharmacopoeia Committee) Pharmacopoeia of the Peoples Republic of China (I) Beijing: Chemical Industry Press; 2005. p. 43. (in Chinese) [Google Scholar]
- 6.Dai HF, Zhou J, Peng ZG, Tan NH. Studies on the chemical constituents of Schisandra chinensis . Nat Prod Res Dev. 2001;13(1):24–26. (in Chinese) [Google Scholar]
- 7.García-Romero E, Pérez-Coello MS, Sanz J, Cabezudo MD. Quantitative analysis of the principal volatile compounds in oak wood by direct thermal desorption (DTD) and GC/MS. Analusis. 1998;26(1):33–34. doi: 10.1051/analusis:1998107. [DOI] [Google Scholar]
- 8.Hou YC, Ching H, Chao PDL, Tsai SY, Wen KC, Hsieh PH, Hsiu SL. Effects of glucose, fructose and 5-hydroxymethyl-2-furaldehyde on the presystemic metabolism and absorption of glycyrrhizin in rabbits. J Pharmacy Pharmacol. 2005;57(2):247–251. doi: 10.1211/0022357055281. [DOI] [PubMed] [Google Scholar]
- 9.Janzowski C, Glaab V, Sammimi E, Schlatte RJ, Eisenbrand G. 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chemical Toxicol. 2000;38(9):801–809. doi: 10.1016/S0278-6915(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 10.Khan QA, Shamsi FA, Hadi SM. Mutagenicity of furfural in plasmid DNA. Cancer Lett. 1995;89(1):95–99. doi: 10.1016/0304-3835(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 11.Li YH, Lu XY. Investigation on the origin of 5-HMF in Shengmaiyin decoction by RP-HPLC method. J Zhejiang Univ Sci B. 2005;6(10):1015–1021. doi: 10.1631/jzus.2005.B1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Zhang XM, Lu Y, Yu GQ, Lu JP, Nan QX, Chen LJ. Browning-inhibition technology for low-lactose milk. Chin Dairy Industry. 2004;32(6):20–23. (in Chinese) [Google Scholar]
- 13.Lo Coco F, Valentini C, Novelli V, Ceccon L. Liquid chromatographic determination of 2-furaldehyde and 5-hydroxymethyl-2-furaldehyde in beer. Anal Chim Acta. 1995;306(1):57–64. doi: 10.1016/0003-2670(94)00675-C. [DOI] [Google Scholar]
- 14.Lu G, Cao W, Zheng JB. Effects of thermal treatment on 5-hydromethyl-2-furaldehyde (HMF) contents in different floral honeys. J Northwest Univ (Nat Sci Ed) 2006;36(2):253–256. (in Chinese) [Google Scholar]
- 15.Miyazawa T, Funazukuri T. Noncatalytic hydrolysis of guar gum under hydrothermal conditions. Carbohydr Res. 2006;341(7):870–877. doi: 10.1016/j.carres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa M, Anzai J, Fujioka J, Isikawa Y. Insecticidal compounds against Drosophila melanogaster from Cornus officinalis Sieb. et Zucc. Nat Prod Res. 2003;17(5):337–339. doi: 10.1080/1057563031000072587. [DOI] [PubMed] [Google Scholar]
- 17.Morales FJ, Jimenez PS. HMF formation during heat-treatment of milk-type products as related to milkfat content. J Food Sci. 1999;64(5):855–859. doi: 10.1111/j.1365-2621.1999.tb15927.x. [DOI] [Google Scholar]
- 18.Nozal MJ, Bernal JL, Toribio L, Jimenez JJ, Martin MT. High-performance liquid chromatographic determination of methyl anthranilate, hydroxymethylfurfural and related compounds in honey. J Chromatogr A. 2001;917(1-2):95–103. doi: 10.1016/S0021-9673(01)00702-6. [DOI] [PubMed] [Google Scholar]
- 19.Palma M, Taylor LT. Supercritical fluid extraction of 5-hydroxymethyl-2-furaldehyde from raisins. J Agric Food Chem. 2001;49(2):628–632. doi: 10.1021/jf001070s. [DOI] [PubMed] [Google Scholar]
- 20.Pamplona R, Bellmunt MJ, Portero M, Riba D, Prat J. Chromatographic evidence for amadori product formation in rat liver aminophospholipids. Life Sci. 1995;57(9):873–879. doi: 10.1016/0024-3205(95)02020-J. [DOI] [PubMed] [Google Scholar]
- 21.Poll L. The influence of apple ripeness and juice storage temperature on the sensory evaluation and composition (volatile and non-volatile components) of apple juice. Lebensm Wiss Technol. 1985;18(4):205–211. [Google Scholar]
- 22.Roig MG, Bello JF, Rivera ZS, Kennedy JF. Studies on the occurrence of non-enzymatic browning during storage of citrus juice. Food Res Inter. 1999;32(9):609–619. doi: 10.1016/S0963-9969(99)00128-3. [DOI] [Google Scholar]
- 23.Sharma VK, Choi J, Sharma N, Choi M, Seo SY. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata . Phytother Res. 2004;18(10):841–844. doi: 10.1002/ptr.1428. [DOI] [PubMed] [Google Scholar]
- 24.Xu BD, Chen K, Lin WJ, Zhang HL. Comparative study on chemical components of supercitical extracts from Herba Ephedrae and honey-prepared Herba Ephedrae. J Guangzhou Univ TCM. 2004;21(3):211–212. (in Chinese) [Google Scholar]
- 25.Zhu DN, Li ZM, Yan YQ, Zhu JG. A research on chemical dynamic changes and drug efficacy of SMS compound prescription chemical researches on SMS prescription (II) Chin J Chin Material Med. 1998;23(5):291–293. (in Chinese) [PubMed] [Google Scholar]
- 26.Zhu DN, YAN YQ, LI ZM. A research on chemical dynamic changes and drug efficacy of SMS compound prescription chemical researches on SMS prescription (III) Chin J Chin Material Med. 1998;23(8):483–485. (in Chinese) [PubMed] [Google Scholar]