Abstract
One of the loci responsible for genetic susceptibility to insulin-dependent diabetes mellitus (IDDM) is the insulin-linked polymorphic region (ILPR, also known as IDDM2). This polymorphic G-rich minisatellite, located in the promoter region of the human insulin gene, comprises a variable number of tandemly repeating sequences related to ACAGGGGTGTGGGG. An interesting characteristic of the ILPR is its ability to form unusual DNA structures in vitro, presumably through formation of G-quartets. This ability to form G-quartets raises the intriguing possibility that transcriptional activity of the insulin gene may in fact be influenced by the quaternary DNA topology of the ILPR. We now show that single nucleotide differences in the ILPR known to affect insulin transcription are correlated with ability to form unusual DNA structures. Through the design and testing of two high transcriptional activity ILPR repeats, we demonstrate that both inter- and intramolecular G-quartet formation in the ILPR can influence transcriptional activity of the human insulin gene, and thus, may contribute to that portion of diabetes susceptibility attributed to the IDDM2 locus.
Keywords: G-quartets, tetrastrand DNA, variable number of tandem repeats, insulin gene-linked polymorphic region
DNA tetraplexes, otherwise known as DNA quadruplexes, G-4 DNA, or G-quartets, are four-stranded DNA structures formed by nucleic acids containing guanine tracts. Readily formed under physiological conditions, these four-stranded DNA structures are highly stabilized by planar arrays of Hoogsteen-bonded guanine quartets (1–5). Although G-quartets have thus far been studied only in vitro, these DNA tetraplexes are attracting increasing attention because of their postulated involvement in a variety of biological processes. For example, telomeric G-quartets, postulated to exist at the ends of human chromosomes (6, 7), are now attractive targets in developing new potent and specific antitumor agents (8). Aside from being potential “anti-cancer knots,” G-quartets are also linked to site-specific genetic recombination in immunoglobulin switch regions (9), the dimerization of HIV RNA (10), and L1 retroposition (11). More recently, DNA quadruplexes have been associated with the promoter regions of DNA, such as the triplet repeat sequence that causes fragile-X syndrome (12–14), the retinoblastoma susceptibility genes (15), and the chicken β-globulin gene (16), raising tantalizing possibilities of controlling gene expression with G-quartets.
One well-known G-rich region is the insulin gene-linked polymorphic region (ILPR), located 363 bp upstream of the human insulin gene (17). The ILPR is composed of a variable number of tandemly repeating sequences related to ACAGGGGTGTGGGG (18). Consequently, the ILPR is commonly referred to in the literature as the VNTR (variable number of tandem repeats). Eleven different variants have been identified, named “a” through “k” (ref. 17; ¶) and tested individually for transcriptional activity with the transcription factor Pur-1 (also known as MAZ and ZF87), a zinc-finger protein that binds to the purine-rich GAGA box of the rat insulin I and II promoters, as well as the ILPR (19–21). Seemingly minor nucleotide variations in this sequence result in wide ranges of transcriptional activity (22). Because of its G-rich nature, the ILPR has long been suspected of adopting non-Watson-Crick base-paired DNA structures. Indeed, biochemical studies have shown that the most common ILPR repeat, a, is capable of forming G-quartets in vitro (23, 24); more recently, spectroscopic studies have provided a detailed look at the stereochemistry of intramolecular G-quartets in repeat a (25). As repeat a is correlated with the highest transcriptional activity (22), we hypothesized that less common repeats such as b and c, which have low transcriptional activities in vivo, may have a lower propensity for forming quadruplex DNA structures in vitro. In attempting to make a simple correlation between in vitro structure of the ILPR and its function in vivo, we found the picture to be more complicated than originally thought, because of multiple pathways for G-quartet formation. Through the design and synthesis of two high transcriptional activity ILPR repeats, transfections, and gel-shift assays, we demonstrate that both inter- and intramolecular G-quartet formation in the ILPR can influence transcriptional activity of the human insulin gene.
Materials and Methods
Plasmids.
Constructs containing tetramers of individual polymorphic repeats or variants were made by synthesizing top and bottom oligonucleotides containing four contiguous homomeric repeats with BamHI/BglII cohesive termini. The top and bottom strands were annealed and cloned upstream of the minimal prolactin promoter–luciferase construct, Prl-luc, as described (19). The Pur-1 cDNA was cloned into the cytomegalovirus (CMV) expression vector pBAT7 (26). All constructs were verified by DNA sequence analysis before use.
Transcriptional Activity of Polymorphic Repeats and Their Compensation Mutants.
Luciferase activity and total protein concentrations from HeLa cells transfected with 10 μg of the reporter plasmid with or without 5 μg of the expression plasmid by the calcium phosphate technique were analyzed as described (22).
Native Gel Electrophoresis Assay for Intramolecular Interactions of Polymorphic Repeats a, b, Variant b, c, and Variant c.
For each polymorphic repeat, eight oligonucleotides were synthesized, containing 0.5–4.0 copies of the respective repeat, in increments of 0.5 repeat. Thus for repeat a, the sequence of oligonucleotide 1 is ACAGGGG, oligonucleotide 2 is ACAGGGGTGTGGGG, oligonucleotide 3 is ACAGGGGTGTGGGGACAGGGG, and so forth, up to oligonucleotide 8, which contains four complete copies of the 14-bp repeat a. Single-stranded oligonucleotides were labeled with [γ-32P]ATP and incubated in 10 mM Tris⋅HCl, pH 7.8/1 mM EDTA, in the presence or absence of 100 mM NaCl at room temperature for 20 min. Samples were loaded on a 20% polyacrylamide gel (19:1 acrylamide/bisacrylamide) cast with or without 100 mM NaCl, and run in 0.5× Tris/borate/EDTA buffer at 4°C such that the rate of migration of the bromophenol blue dye was no more than 1.0 cm/h. Gels were transferred to Whatman filter paper, dried, and analyzed by autoradiography.
Native Gel Electrophoresis Assay for Intermolecular Interactions in Polymorphic Repeats a, b, Variant b, c, and Variant c.
Oligonucleotides containing 1.5 repeats (21-mer) of a, b, variant b, c, and variant c were synthesized. For each repeat, 1 μg of [γ-32P]ATP-labeled single-stranded oligonucleotide in a final volume of 10 μl was incubated in 1 mM EDTA/50 mM NaCl at 95°C for 2 min, and then at 65°C for 72 h as described (27). Samples were loaded on an 8% polyacrylamide gel (29:1 acrylamide/bisacrylamide), cast with 50 mM NaCl, and run in 0.5× Tris/borate/EDTA buffer at 4°C. The gels were transferred to Whatman filter paper, dried, and analyzed by autoradiography.
Methylation Analysis of Guanine Bases.
Gels similar to the one shown in Fig. 2a were run and analyzed by autoradiography while still wet. Bands corresponding to either monomer or higher-order complexes in the a repeat were excised. The excised DNA was recovered by elution, incubated with dimethyl sulfate (DMS), and subjected to cleavage with piperidine as described (28).
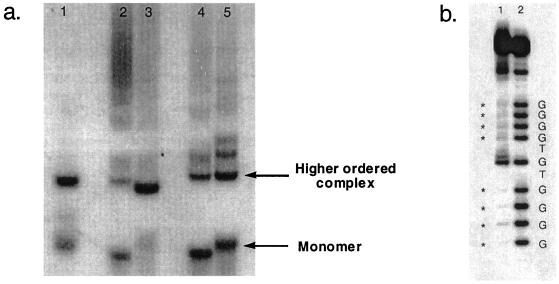
Figure 2.
(a) Assaying for intermolecular interactions. Lane 1, repeat a; lane 2, repeat b; lane 3, repeat variant b; lane 4, repeat c; lane 5, repeat variant c. (b) Methylation analysis of the guanine residues in repeat a. Lane 1, higher-order complex of a single repeat of a. Guanines inaccessible to DMS are marked with asterisks. Lane 2, monomer form of a single repeat of a.
Pur-1 Translation Using Rabbit Reticulocyte Lysate.
Pur-1 was subcloned into a pBAT9 expression vector and translated by using the methods and materials provided in Promega's rabbit reticulocyte lysate kit. The translated Pur-1 was then incubated with 0.5, 1.0, 2.0, or 4.0 repeats of the single-stranded [γ-32P]ATP-labeled repeat a in 10 mM Tris⋅HCl, pH 7.8/1 mM EDTA/100 mM NaCl at room temperature for 20 min. The individual protein/DNA mixtures were then loaded on a 6% polyacrylamide gel cast with 100 mM NaCl and run in 0.5× Tris/borate/EDTA buffer at 4°C. The gel was then transferred to Whatman filter paper, dried, and analyzed by autoradiography.
Results and Discussion
We previously determined that single nucleotide differences among the 11 ILPR repeats affected their ability to bind to the transcription factor Pur-1 in vitro and drastically affected their transcriptional activity in vivo (22). The most common repeat, a, has the highest transcriptional activity, and the two linker regions, 5′ and 3′ to the G residues, show Watson–Crick (WC) complementarity. We noted that repeats b and c, which have low transcriptional activity, contain linker regions that lack WC complementarity. To explore the possibility that WC base-pairing facilitates the formation of a secondary DNA structure—e.g., G-quartets—and thus may influence transcription, we synthesized several variants (Table 1) that potentially can restore WC formation of the linker regions. In repeat b, we made a single nucleotide change in the first linker region from ACA to AGA, which allows a potential WC base pair with the second linker region TCT. Likewise, in repeat c, we changed the ACA of the first linker region to AGGA so that it could potentially form WC base pairs with the second linker TCCT. We tested these nucleotide variants (variant b and variant c) in transient transfection assays using full-length human Pur-1, and found that the sequence changes we introduced resulted in a striking recovery of transcriptional activity, comparable to levels seen with the a repeat. These data support the hypothesis that WC base-pairing might favor a secondary structure that influences insulin transcription through preferential recognition by Pur-1.
Table 1.
Polymorphic repeat sequences of the ILPR with their corresponding transcriptional activity
| Polymorphic repeat | Sequence | Transcriptional activity, % |
|---|---|---|
| a | ACA GGGG TGT GGGG | 100 |
| b | ACA GGGG TCT GGGG | 30.0 ± 0.4 |
| Variant b | AGA GGGG TCT GGGG | 99.90 ± 0.04 |
| c | ACA GGGG TCCT GGGG | 20.0 ± 0.4 |
| Variant c | AGGA GGGG TCCT GGGG | 100.00 ± 0.004 |
“Restored” nucleotides are marked in bold-face. Constructs for the transfection studies were made by cloning a synthetic oligonucleotide containing four tandem copies of the corresponding polymorphic repeats upstream of the minimal prolactin-luciferase construct, Prl-luc. The transcriptional activity was determined in HeLa cells transfected in the absence and presence of a Pur-1 expression vector driven by a cytomegalovirus (CMV) promoter as described (19). Transcriptional activities of the a repeat are set arbitrarily at 100% as described in ref. 22.
DNA tetraplexes are formed under physiological salt and pH conditions from guanine-rich DNA. Different G-quartet isomers form, depending on the DNA strand concentration and cations present (29, 30). Intermolecular G-quartets arise from the association of four individual single-stranded G-rich DNA molecules, whereas intramolecular G-quartets are formed from a single strand of G-rich DNA. A combination of intramolecular and intermolecular interactions can result from the dimerization of two DNA molecules, each containing two G-rich motifs. We examined the ability of repeats a, b, and c and variants b and c to form both intra- and intermolecular G-quartet structures.
Intramolecular Interactions of the ILPR Repeats Are Correlated with Their Transcriptional Activity.
Gupta and colleagues (25) have shown through spectroscopic studies that a single polymorphic repeat a is capable of forming an intramolecularly folded G-quartet. However, it is unknown whether multiple contiguous tandem repeats as they occur in vivo are also capable of forming intramolecular G-quartets. If so, it is conceivable that intramolecular secondary structure at the ILPR might influence transcriptional activity.
To test whether ILPR repeats can form intramolecular G-quartets, we performed gel-based hairpin assays. In the absence of salt, synthetic single-stranded oligonucleotides with increasing numbers of polymorphic repeat a migrate strictly according to length (Fig. 1a). However, in the presence of NaCl at a physiological concentration, which is known to stabilize G-quartet DNA, oligonucleotides containing four runs of guanines (i.e., two polymorphic repeats) exhibit gel mobilities that are greater than expected, consistent with an intramolecularly folded, more compact G-quartet structure (Fig. 1b, lanes 4–8). These data suggest that multiple repeats of a as they occur in the insulin gene are also capable of forming compact G-quartet structures, provided that there is a mechanism for creating single-stranded DNA at that locus.
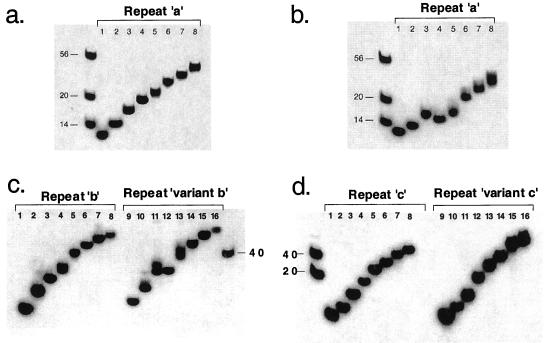
Figure 1.
Assaying for intramolecular interactions. (a) Nondenaturing 20% PAGE of 32P-labeled repeat a in the absence of salt. The first lane contains the 14-, 20-, and 56-mer markers. The rest of the eight lanes labeled 1–8 contain 0.5–4.0 copies of repeat a in increments of 0.5 repeat. For example, lane 1, 0.5 repeat, ACAGGGG; lane 2, 1.0 repeat, ACAGGGGTGTGGGG; etc. (b) Same as a, but in the presence of 100 mM NaCl. (c) Same as for b except lanes 1–8 are repeats b and lanes 9–16 are repeats variant b. (d) Same as for b except lanes 1–8 are repeats c and lanes 9–16 are repeats variant c.
Having established that tandem a repeats clearly form intramolecular hairpins through guanine–guanine interactions, we addressed the question of whether repeats b and c and their corresponding variants do the same. Our experiments clearly demonstrate that repeats b and c lack the same propensity for forming intramolecular hairpins as repeat a (Fig. 1 c and d, lanes 1–8). This finding could explain why repeats b and c have low transcriptional activity in transcription assays. If this hypothesis is correct, the variants, which show full restoration of transcriptional activity, should exhibit the ability to form intramolecular hairpins, similar to repeat a. The results show a marked difference between variant b (Fig. 1c, lanes 9–16) and variant c (Fig. 1d, lanes 9–16). The ability to form intramolecular hairpins has been restored in variant b, suggesting that intramolecular G-quartets may indeed play a role in transcription. In contrast, variant c does not appear to form intramolecular G-quartets, which indicates that something else must play a role in its high transcriptional activity. Because previous studies have suggested that the ILPR might form higher-order complexes through intermolecular G-quartet interactions (23, 24, 31, 32), we examined this possibility in greater detail below.
Intermolecular Interactions of the ILPR Repeats also Correlate with Their Transcriptional Activity.
To address the possibility that the restored transcriptional activities we observed in vivo for variant b and variant c resulted from WC-stabilized intermolecular G-quartets (23), we turned to in vitro studies of the individual ILPR repeat molecules. First we assessed the ability of these molecules to form intermolecular associations. We synthesized single-stranded 21-mer oligonucleotides containing 1.5 repeats of a, b, variant b, and variant c, and then incubated them at high temperature for various lengths of time to allow formation of intermolecular higher-order complexes. These complexes are easily resolved by native gel electrophoresis (27). We chose an oligonucleotide length of 1.5 repeats because it lacks the runs of guanines sufficient to form intramolecular G-quartets, the formation of which might interfere with the identification of intermolecular tetrastrand DNA formation. Fig. 2a shows that the a repeat forms two predominant species, one corresponding to a monomer and the other corresponding to a higher-order complex consisting of an unknown number of molecules. We suspect that this complex consists of two or more DNA molecules bonded intermolecularly through G–G interactions and/or WC base-pairing of the linker regions. Significantly, the higher-order intermolecular complex observed with the a repeat is also observed with the high transcriptional variant b (lane 3) and variant c (lane 5), but is much less prevalent with the low transcriptional wild-type repeat b (lane 2) and c (lane 4). We suspected that the nucleotide sequence changes we made in repeats b and c restore WC base-pairing in the linker regions, and consequently facilitate the formation of higher-order WC-stabilized complexes.
To assay whether the intermolecular associations observed for repeats a, variant b, and variant c indeed occur through G–G base-pairing, we subjected a single repeat of a, 5′-ACAGGGGTGTGGGG, to conditions similar to those in Fig. 2a. We isolated the DNA corresponding to either the monomer or higher-order complexes (Fig. 2a, lower and upper arrows, respectively), and probed the accessibility of guanine bases with DMS. This methylation analysis of the guanine residues shows that the runs of four Gs in the intermolecular complex (Fig. 2b, lane 1) are inaccessible to the methylating reagent DMS, indicating that those guanines are involved in Hoogsteen base-pairing through the N7. In contrast, all of the guanines in the monomer form of repeat a are accessible to DMS (Fig. 2b, lane 2). These data suggest that the intermolecular associations observed for repeat a are most likely occurring through G-quartet formation from four individual DNA molecules. Taken together with the transcriptional data (Table 1), these in vitro experiments suggest that the ability of polymorphic repeats in the ILPR to form intermolecular G-quartets may be important in transcriptional activation.
Pur-1 Binds to Single-Stranded Oligonucleotides Capable of Forming G-Quartets.
We previously demonstrated that transcriptional activity of the ILPR was correlated with Pur-1 binding (22). Taking into consideration the unusual structure of the ILPR in vitro, we hypothesized that perhaps Pur-1 recognizes the quaternary DNA structure of the ILPR, as opposed to a simple contiguous stretch of nucleotides. This hypothesis is difficult to prove without in vivo probes for DNA quartets whose assay is independent of protein occupancy of DNA. Despite this technical limitation, it should be possible to make a correlation between the ability of a DNA sequence to form G-quartets and its binding affinity to a given transcription factor. If our hypothesis is correct, Pur-1 should bind to polymorphic repeats that form G-quartets (either intra- or intermolecular), but should not bind to those that do not form G-quartets. As we observed in Fig. 1, polymorphic repeats with only one or two runs of Gs do not form intramolecular G-quartets under physiological salt conditions, but repeats with four runs of Gs (or greater) readily form intramolecular G-quartets under these conditions. To determine whether Pur-1 preferentially binds to oligonucleotides capable of forming G-quartets, we translated Pur-1 in rabbit reticulocyte lysate, and incubated it with 0.5, 1.0, 2.0, or 4.0 repeats of single-stranded polymorphic repeat a in the presence of 100 mM NaCl. Gel analysis shows that Pur-1 does not bind to repeat a in which only 1 or 2 stretches of Gs are present (Fig. 3, lanes 1–2), nor does it bind to an unrelated double- and single-stranded DNA (Fig. 3, lane 5). However, Pur-1 does bind to repeat a when four or eight runs of Gs are present, as can be seen by the single and double upper bands (Fig. 3, lanes 3 and 4), most likely indicating a monomer and dimer of Pur-1, respectively. These results are consistent with a mechanism in which Pur-1 recognizes a G-quartet structure in ILPR DNA.
Figure 3.
Pur-1 binds to oligonucleotides capable of forming G-quartets. Lane 1, 0.5 repeat of a; lane 2, 1.0 repeat of a; lane 3, 2.0 repeats of a; lane 4, 4.0 repeats of a; lane 5, control containing nonspecific double- and single-stranded DNA.
Pur-1, G-Quartet Structures, and Diabetes Susceptibility.
Diabetes susceptibility has both genetic and environmental components. The genetic component of diabetes susceptibility is in itself complex, attributable to at least 14 distinct genetic loci, including the ILPR (also referred to as IDDM2). We have shown here that both intra- and intermolecular G-quartet formation of the ILPR is important for transcription by Pur-1. Of 11 naturally occurring repeats, only repeats a, e, h, and k show high to moderate transcriptional activity. By making compensatory changes in the repeats with low transcriptional activity, we have identified ILPR repeats with high transcriptional activities. These high transcriptional repeats form either inter or intramolecular G-quartets, or both, whereas their low transcriptional activity counterparts fail to form either of these unusual structures.
We hypothesize that Pur-1 modulates insulin transcription through the recognition of both inter- and intramolecular G-quartets formed by the ILPR. For most of the repeats, activation by Pur-1 in vivo correlates with Pur-1 binding in vitro. However, with some repeats, Pur-1 binding is not correlated with transcriptional activation (22), suggesting that other proteins may interact with Pur-1 to modulate activity of the ILPR. These factors may influence insulin transcription directly through interaction with the transcriptional machinery, or indirectly, by modulating the quaternary structure of ILPR DNA, which may in turn influence the binding of structure-specific DNA-binding proteins. To this end, we have screened an insulinoma cDNA library for ILPR-binding proteins and have identified proteins with virtually mirror-image binding properties of those shown by Pur-1, namely proteins which preferentially bind to low transcriptional activity repeats (A.L and G.C.K., unpublished data). Our present work suggests that the quaternary structure of the ILPR plays a role in transcriptional activity of the ILPR, whereas our more recent discovery of a spectrum of ILPR-binding proteins suggests a complex interplay of activators and inhibitors, whose own levels may determine the final balance of ILPR activity. One or more of these proteins could play a role in pathways encoded by the as-yet-unidentified genes in other diabetes susceptibility loci (IDDM3 through IDDM14) (33). Further work will be necessary to determine whether any of these proteins simply interact with DNA once the structures have been formed, or whether they play an active role in mediating the formation of these structures.
Acknowledgments
We thank Suzanne Seavello for expert technical assistance, Monica Sauer and Michael German for helpful suggestions, and Thomas Ryder for critical reading of the manuscript.
Abbreviations
- ILPR
insulin gene-linked polymorphic region
- DMS
dimethyl sulfate
- WC
Watson–Crick
- VNTR
variable number of tandem repeats
Footnotes
The inconsistency in the literature over the definition of the ILPR repeat units b and c is probably caused by a clerical error in the upper panel of table 2 in ref. 17. Analysis of the sequence for the 5′ flanking region of the human insulin gene in figure 1 of ref. 17 with the nomenclature used in the lower panel of table 2 in ref. 17 indicates that the unit definitions for repeats b and c are interchanged in the upper panel of that table 2.
References
- 1.Guschlbauer W, Chantof J F, Thiele D. J Biomol Struct Dyn. 1990;8:491–511. doi: 10.1080/07391102.1990.10507825. [DOI] [PubMed] [Google Scholar]
- 2.Sen D, Gilbert W. Curr Opin Struct Biol. 1991;1:435–438. [Google Scholar]
- 3.Sundquist W I. Nucleic Acids Mol Biol. 1991;5:1–24. [Google Scholar]
- 4.Williamson J R. Curr Opin Struct Biol. 1993;3:357–362. [Google Scholar]
- 5.Williamson J R. Annu Rev Biophys Biomol Struct. 1994;23:703–730. doi: 10.1146/annurev.bb.23.060194.003415. [DOI] [PubMed] [Google Scholar]
- 6.Williamson J R, Raghuraman M K, Cech T R. Cell. 1989;59:871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn E H. Nature (London) 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 8.Haq I, Trent J O, Chowdhry B Z, Jenkins T C. J Am Chem Soc. 1999;121:1768–1779. [Google Scholar]
- 9.Sen D, Gilbert W. Nature (London) 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 10.Sundquist W I, Heapy S. Proc Natl Acad Sci USA. 1993;90:3393–3397. doi: 10.1073/pnas.90.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell R, Usdin K. Mol Biol Evol. 1997;14:144–155. doi: 10.1093/oxfordjournals.molbev.a025747. [DOI] [PubMed] [Google Scholar]
- 12.Fry M, Leob L A. Proc Natl Acad Sci USA. 1994;91:4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadel Y, Weismanshomer P, Fry M. J Biol Chem. 1995;270:28970–28977. doi: 10.1074/jbc.270.48.28970. [DOI] [PubMed] [Google Scholar]
- 14.Fry M, Loeb L A. J Biol Chem. 1999;274:12797–12802. doi: 10.1074/jbc.274.18.12797. [DOI] [PubMed] [Google Scholar]
- 15.Murchie A I, Lilley D M. Nucleic Acids Res. 1992;20:49–53. doi: 10.1093/nar/20.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell R M, Woodford K J, Weitzmann M N, Usdin K. J Biol Chem. 1996;271:5208–5214. doi: 10.1074/jbc.271.9.5208. [DOI] [PubMed] [Google Scholar]
- 17.Bell G I, Selby M, Rutter W J. Nature (London) 1982;295:31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- 18.Rotwein P, Yokoyama S, Didier D K, Chirgwin J M. Am J Hum Genet. 1986;39:291–299. [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy G C, Rutter W J. Proc Natl Acad Sci USA. 1992;89:11498–11502. doi: 10.1073/pnas.89.23.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy G C, Rutter W J. Biochem Soc Trans. 1993;21:178–180. doi: 10.1042/bst0210178. [DOI] [PubMed] [Google Scholar]
- 21.Bossone S A, Asselin C, Patel A J, Marcu K B. Proc Natl Acad Sci USA. 1992;89:7452–7456. doi: 10.1073/pnas.89.16.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy G C, German M S, Rutter W J. Nat Genet. 1995;9:293–298. doi: 10.1038/ng0395-293. [DOI] [PubMed] [Google Scholar]
- 23.Hammond-Kosack M C U, Docherty K. FEBS Lett. 1992;301:79–82. doi: 10.1016/0014-5793(92)80214-2. [DOI] [PubMed] [Google Scholar]
- 24.Hammond-Kosack M C U, Dobrinski B, Lurz R, Docherty K, Kilpatrick M W. Nucleic Acids Res. 1992;20:231–236. doi: 10.1093/nar/20.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catasti P, Chen X, Moyzis R K, Bradbury E M, Gupta G. J Mol Biol. 1996;264:534–545. doi: 10.1006/jmbi.1996.0659. [DOI] [PubMed] [Google Scholar]
- 26.German M S, Wang J, Chadwick R B, Rutter W J. Genes Dev. 1992;6:2165–2176. doi: 10.1101/gad.6.11.2165. [DOI] [PubMed] [Google Scholar]
- 27.Sen D, Gilbert W. Nature (London) 1990;344:410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 28.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. Vol. 2. New York: Greene; 1994. p. 12.3. [Google Scholar]
- 29.Hardin C C, Watson T, Corregan M, Bailey C. Biochemistry. 1992;31:833–841. doi: 10.1021/bi00118a028. [DOI] [PubMed] [Google Scholar]
- 30.Aboul-ela F, Murchie A I H, Lilley D M J. Nature (London) 1992;360:280–282. doi: 10.1038/360280a0. [DOI] [PubMed] [Google Scholar]
- 31.Hammond-Kosack M C U, Kilpatrick M W, Docherty K. J Mol Endocrinol. 1992;9:221–225. doi: 10.1677/jme.0.0090221. [DOI] [PubMed] [Google Scholar]
- 32.Hammond-Kosack M C U, Kilpatrick M W, Docherty K. J Mol Endocrinol. 1993;10:121–126. doi: 10.1677/jme.0.0100121. [DOI] [PubMed] [Google Scholar]
- 33.Owerbach D, Gabbay K H. Diabetes. 1996;45:544–551. doi: 10.2337/diab.45.5.544. [DOI] [PubMed] [Google Scholar]