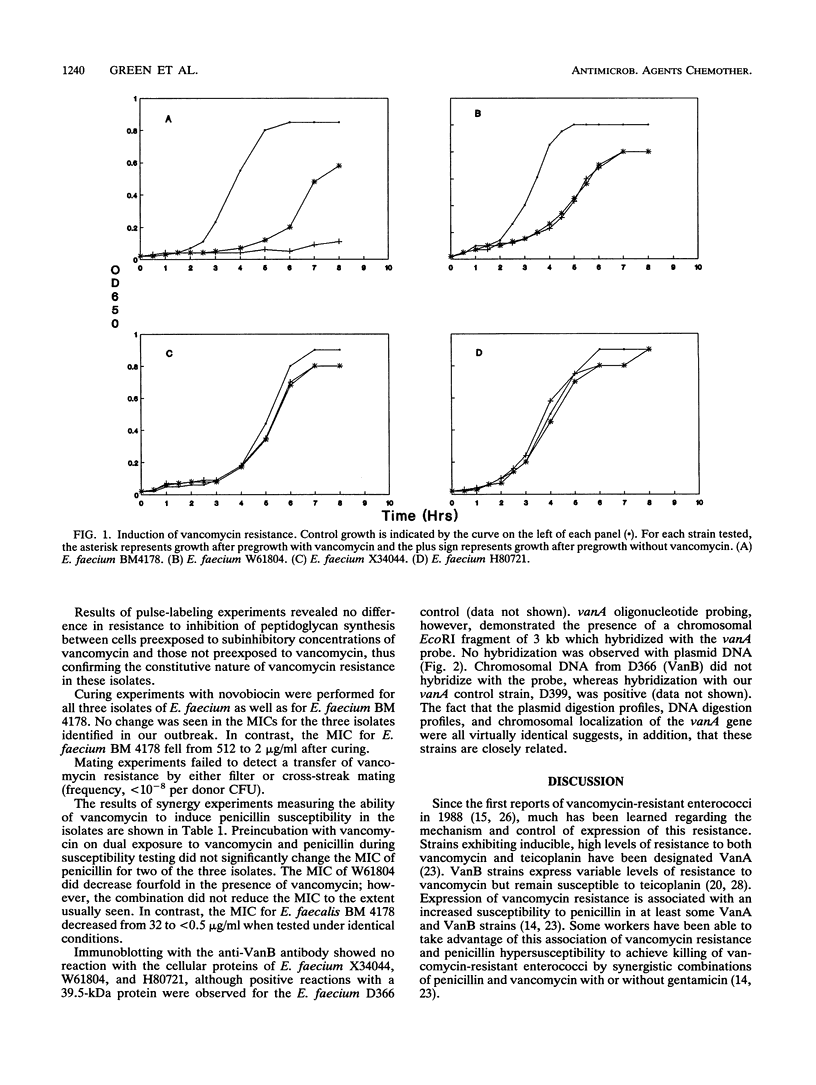
Abstract
Vancomycin resistance among enterococci has recently been recognized. Synergy between vancomycin and penicillin has been shown in vitro for isolates of Enterococcus faecium resistant to both of these antibiotics. We describe three isolates of vancomycin-resistant E. faecium which demonstrate unique phenotypic characteristics. The isolates exhibited high-level resistance to both vancomycin and teicoplanin, consistent with the VanA phenotype. However, resistance in these isolates could not be induced or cured, and mating experiments failed to detect a transfer of resistance. The combination of vancomycin and penicillin did not significantly change the MIC of penicillin for any of the three isolates. Immunoblotting with polyclonal anti-VanB antibody showed no reaction with the cellular proteins of these strains. Probing with a vanA oligonucleotide revealed hybridization with chromosomal but not plasmid DNA. The mechanism of constitutive resistance of those strains remains unclear. A second mutational change, perhaps involving PBP 5, may explain the presence of resistance to synergistic combination penicillin-vancomycin therapy. In vitro evaluation of penicillin-vancomycin should be carried out in all clinical cases where this therapeutic regimen is being considered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisson-Noël A., Dutka-Malen S., Molinas C., Leclercq R., Courvalin P. Cloning and heterospecific expression of the resistance determinant vanA encoding high-level resistance to glycopeptides in Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1990 May;34(5):924–927. doi: 10.1128/aac.34.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Wright G. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991 Oct 29;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. The VANA glycopeptide resistance protein is related to D-alanyl-D-alanine ligase cell wall biosynthesis enzymes. Mol Gen Genet. 1990 Dec;224(3):364–372. doi: 10.1007/BF00262430. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Clewell D. B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987 Aug;169(8):3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R., Hollis D., Collins M. D. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989 Apr;27(4):724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraimow H. S., Venuti E. Inconsistent bactericidal activity of triple-combination therapy with vancomycin, ampicillin, and gentamicin against vancomycin-resistant, highly ampicillin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1992 Jul;36(7):1563–1566. doi: 10.1128/aac.36.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wadowsky R. M., Barbadora K. Recovery of vancomycin-resistant gram-positive cocci from children. J Clin Microbiol. 1990 Mar;28(3):484–488. doi: 10.1128/jcm.28.3.484-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Perlman D. C., Altarac D., McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis. 1992 Mar;14(3):655–661. doi: 10.1093/clinids/14.3.655. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., Vellozzi E. M., Shapiro J., Rubin L. G. Clinical laboratory challenges in the recognition of Leuconostoc spp. J Clin Microbiol. 1988 Mar;26(3):479–483. doi: 10.1128/jcm.26.3.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Bingen E., Su Q. H., Lambert-Zechovski N., Courvalin P., Duval J. Effects of combinations of beta-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):92–98. doi: 10.1128/aac.35.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Boisivon A., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecium, D399. J Antimicrob Chemother. 1989 Apr;23(4):503–508. doi: 10.1093/jac/23.4.503. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Etter L., Gutmann L. Synergistic killing of vancomycin-resistant enterococci of classes A, B, and C by combinations of vancomycin, penicillin, and gentamicin. Antimicrob Agents Chemother. 1991 Apr;35(4):776–779. doi: 10.1128/aac.35.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Marino J., Jacobs M. R. Infection caused by vancomycin-resistant Streptococcus sanguis II. Antimicrob Agents Chemother. 1984 Apr;25(4):527–528. doi: 10.1128/aac.25.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Courvalin P. Conjugative plasmid transfer from Enterococcus faecalis to Escherichia coli. J Bacteriol. 1988 Sep;170(9):4388–4391. doi: 10.1128/jb.170.9.4388-4391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Wenocur H. S., Smith M. A., Vellozzi E. M., Shapiro J., Isenberg H. D. Odontogenic infection secondary to Leuconostoc species. J Clin Microbiol. 1988 Sep;26(9):1893–1894. doi: 10.1128/jcm.26.9.1893-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- Williamson R., Calderwood S. B., Moellering R. C., Jr, Tomasz A. Studies on the mechanism of intrinsic resistance to beta-lactam antibiotics in group D streptococci. J Gen Microbiol. 1983 Mar;129(3):813–822. doi: 10.1099/00221287-129-3-813. [DOI] [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Shlaes D. M., Williamson R., Collatz E. Comparison of vancomycin-inducible proteins from four strains of Enterococci. FEMS Microbiol Lett. 1990 Jun 15;58(1):101–105. doi: 10.1016/0378-1097(90)90110-c. [DOI] [PubMed] [Google Scholar]