Abstract
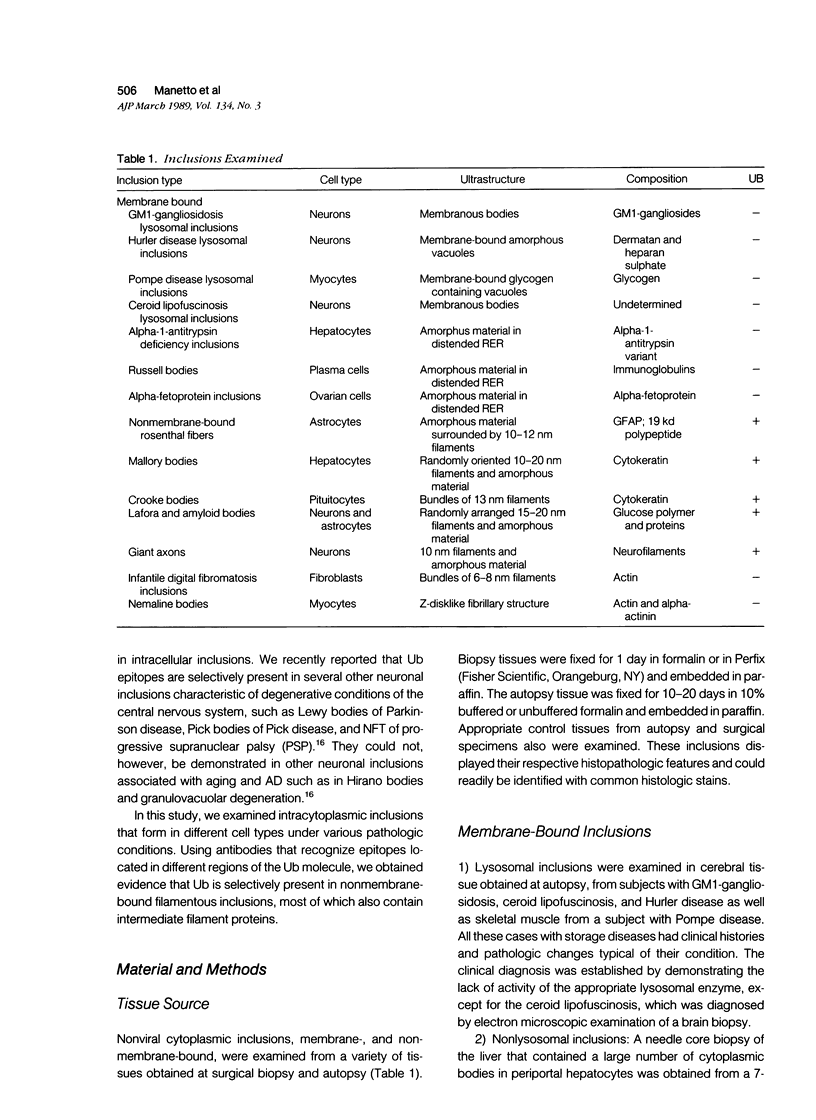
The authors have shown previously that ubiquitin, a protein involved in the degradation of short-lived and abnormal proteins, is present in several cytoplasmic inclusions of neurons. This study used a library of antibodies to ubiquitin and immunohistochemically examined for the presence of ubiquitin in nonviral intracytoplasmic inclusions that form in different cell types under various pathologic conditions. Membrane-bound lysosomal and nonlysosomal inclusions such as those of storage disease, Russell bodies, alpha-1-antitrypsin and alpha-fetoprotein as well as nonmembrane-bound inclusions were examined. Ubiquitin epitopes were detected in some of the nonmembrane-bound inclusions only. The ubiquitin-containing inclusions were the Rosenthal fibers, Mallory bodies, Crooke bodies, Lafora bodies, amyloid bodies, and the giant axons of giant axonal neuropathy. Nemaline bodies and the inclusions of juvenile digital fibromatosis, both of which contain actin and actinbinding proteins, did not show immunoreaction. These findings, as well as those of the previous study, show that the presence of ubiquitin in cellular inclusions is selective. The ubiquitin-containing inclusions are not membrane bound; they are fibrillary and most contain also intermediate filament-related proteins. The role of ubiquitin in the formation of these inclusions remains to be elucidated.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbury A. K., Gale M. K., Cox S. C., Baringer J. R., Berg B. O. Giant axonal neuropathy--a unique case with segmental neurofilamentous masses. Acta Neuropathol. 1972;20(3):237–247. doi: 10.1007/BF00686905. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Velasco M. E., Sipple J., Gambetti P. Immunochemical characterization of antisera to rat neurofilament subunits. J Neurochem. 1981 Nov;37(5):1260–1265. doi: 10.1111/j.1471-4159.1981.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Ball E., Karlik C. C., Beall C. J., Saville D. L., Sparrow J. C., Bullard B., Fyrberg E. A. Arthrin, a myofibrillar protein of insect flight muscle, is an actin-ubiquitin conjugate. Cell. 1987 Oct 23;51(2):221–228. doi: 10.1016/0092-8674(87)90149-8. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Molecular determinants of protein half-lives in eukaryotic cells. FASEB J. 1987 Nov;1(5):349–357. doi: 10.1096/fasebj.1.5.2824267. [DOI] [PubMed] [Google Scholar]
- Foreman R. C. Alpha 1-antitrypsin deficiency--a defect in secretion. Biosci Rep. 1987 Apr;7(4):307–311. doi: 10.1007/BF01121452. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Schmid E., Osborn M., Weber K. Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Lab Invest. 1979 Feb;40(2):207–220. [PubMed] [Google Scholar]
- Fried V. A., Smith H. T., Hildebrandt E., Weiner K. Ubiquitin has intrinsic proteolytic activity: implications for cellular regulation. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3685–3689. doi: 10.1073/pnas.84.11.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway P. G., Grundke-Iqbal I., Iqbal K., Perry G. Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol. 1988 Nov;47(6):654–663. doi: 10.1097/00005072-198811000-00008. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Perry G., Gambetti P. Hirano body filaments contain actin and actin-associated proteins. J Neuropathol Exp Neurol. 1987 Mar;46(2):185–199. doi: 10.1097/00005072-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Gambetti P., Di Mauro S., Hirt L., Blume R. P. Myoclonic epilepsy with lafora bodies. Some ultrastructural, histochemical, and biochemical aspects. Arch Neurol. 1971 Dec;25(6):483–493. doi: 10.1001/archneur.1971.00490060017002. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Corbin E. Isolation of a major protein component of Rosenthal fibers. Am J Pathol. 1988 Mar;130(3):569–578. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Doniach I. Ultrastructure of plasma cells containing Russell bodies in human stomach and thyroid. J Clin Pathol. 1970 Oct;23(7):608–612. doi: 10.1136/jcp.23.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Hazan R., Denk H., Franke W. W., Lackinger E., Schiller D. L. Change of cytokeratin organization during development of Mallory bodies as revealed by a monoclonal antibody. Lab Invest. 1986 May;54(5):543–553. [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J., Freeman J. M., Mathieson G. Light and electron microscopic observations on Rosenthal fibers in Alexander's disease and in multiple sclerosis. J Neuropathol Exp Neurol. 1970 Oct;29(4):524–551. doi: 10.1097/00005072-197010000-00002. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Kikuchi M., Ohtsuki I., Enjoji M., Suenaga N., Mori R. Infantile digital fibromatosis. Identification of actin filaments in cytoplasmic inclusions by heavy meromyosin binding. Cancer. 1983 Nov 1;52(9):1653–1661. doi: 10.1002/1097-0142(19831101)52:9<1653::aid-cncr2820520918>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Khavkin T. More on the Russell body. Am J Clin Pathol. 1984 Jan;81(1):141–142. doi: 10.1093/ajcp/81.1.141. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kinney R. B., Gottfried M. R., Hodson A. K., Autilio-Gambetti L., Graham D. G. Congenital giant axonal neuropathy. Arch Pathol Lab Med. 1985 Jul;109(7):639–641. [PubMed] [Google Scholar]
- Kuzuhara S., Mori H., Izumiyama N., Yoshimura M., Ihara Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 1988;75(4):345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988 May;155(1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Smith H. T., Fried V. A. Ubiquitin is a component of the microtubule network. Proc Natl Acad Sci U S A. 1988 May;85(9):3019–3023. doi: 10.1073/pnas.85.9.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P. E., Horoupian D. S., Goldman J. E., Hess M. A. Cytoplasmic filaments of Crooke's hyaline change belong to the cytokeratin class. An immunocytochemical and ultrastructural study. Am J Pathol. 1984 Aug;116(2):214–222. [PMC free article] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Stewart D., Friedman R., Manetto V., Autilio-Gambetti L., Gambetti P. Filaments of Pick's bodies contain altered cytoskeletal elements. Am J Pathol. 1987 Jun;127(3):559–568. [PMC free article] [PubMed] [Google Scholar]
- Robitaille Y., Carpenter S., Karpati G., DiMauro S. D. A distinct form of adult polyglucosan body disease with massive involvement of central and peripheral neuronal processes and astrocytes: a report of four cases and a review of the occurrence of polyglucosan bodies in other conditions such as Lafora's disease and normal ageing. Brain. 1980 Jun;103(2):315–336. doi: 10.1093/brain/103.2.315. [DOI] [PubMed] [Google Scholar]
- Roukema P. A. Oderkerk CH: Isolation and preliminary characterization of corpora amylacea from human brain. Psychiatr Neurol Neurochir. 1970 Mar-Apr;73(2):87–96. [PubMed] [Google Scholar]
- Sakai M., Austin J., Witmer F., Trueb L. Studies of corpora amylacea. I. Isolation and preliminary characterization by chemical and histochemical techniques. Arch Neurol. 1969 Nov;21(5):526–544. doi: 10.1001/archneur.1969.00480170098011. [DOI] [PubMed] [Google Scholar]
- Schochet S. S., Jr, Lampert P. W., Earle K. M. Alexander's disease. A case report with electron microscopic observations. Neurology. 1968 Jun;18(6):543–549. doi: 10.1212/wnl.18.6.543. [DOI] [PubMed] [Google Scholar]
- Schochet S. S., Jr, McCormick W. F., Halmi N. S. Acidophil adenomas with intracytoplasmic filamentous aggregates. A light and electron microscopic study. Arch Pathol. 1972 Jul;94(1):16–22. [PubMed] [Google Scholar]
- Shultz L. D., Coman D. R., Lyons B. L., Sidman C. L., Taylor S. Development of plasmacytoid cells with Russell bodies in autoimmune "viable motheaten" mice. Am J Pathol. 1987 Apr;127(1):38–50. [PMC free article] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- St John T., Gallatin W. M., Siegelman M., Smith H. T., Fried V. A., Weissman I. L. Expression cloning of a lymphocyte homing receptor cDNA: ubiquitin is the reactive species. Science. 1986 Feb 21;231(4740):845–850. doi: 10.1126/science.3003914. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Perry G., Autilio-Gambetti L., Manetto V., Gambetti P. Influence of neuronal location on antigenic properties of neurofibrillary tangles. Ann Neurol. 1988 Jun;23(6):604–610. doi: 10.1002/ana.410230613. [DOI] [PubMed] [Google Scholar]
- Takashina T., Kanda Y., Hayakawa O., Kudo R., Ito E., Sagae S. Yolk sac tumors of the ovary and the human yolk sac. Am J Obstet Gynecol. 1987 Jan;156(1):223–229. doi: 10.1016/0002-9378(87)90242-0. [DOI] [PubMed] [Google Scholar]
- Thomas C., Love S., Powell H. C., Schultz P., Lampert P. W. Giant axonal neuropathy: correlation of clinical findings with postmortem neuropathology. Ann Neurol. 1987 Jul;22(1):79–84. doi: 10.1002/ana.410220118. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Young R., Sassani J., Ramer J., Horoupian D. S. Alexander's disease: further light-, and electron-microscopic observations. Acta Neuropathol. 1983;61(1):36–42. doi: 10.1007/BF00688384. [DOI] [PubMed] [Google Scholar]
- Velasco M. E., Dahl D., Roessmann U., Gambetti P. Immunohistochemical localization of glial fibrillary acidic protein in human glial neoplasms. Cancer. 1980 Feb;45(3):484–494. doi: 10.1002/1097-0142(19800201)45:3<484::aid-cncr2820450312>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2B can be modified by the attachment of ubiquitin. Nucleic Acids Res. 1980 Oct 24;8(20):4671–4680. doi: 10.1093/nar/8.20.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Robson R. M., Stromer M. H., Dahl D. S., Oda T. Actin filaments form the backbone of nemaline myopathy rods. Nature. 1978 Jan 19;271(5642):265–267. doi: 10.1038/271265a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Robson R. M., Stromer M. H., Dahl D. S., Oda T. Nemaline myopathy rod bodies. Structure and composition. J Neurol Sci. 1982 Oct;56(1):35–56. doi: 10.1016/0022-510x(82)90059-4. [DOI] [PubMed] [Google Scholar]






