Abstract
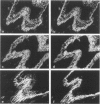
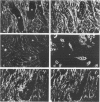
Gelsolin, a Ca2+-sensitive protein present in many mammalian cells, regulates actin filament length by multiple mechanisms. In tumor cells, increased amounts of F-actin without change in its total amount have been observed, suggesting a modification in the organisation of this protein. The authors have localized gelsolin, total actin, alpha-smooth muscle actin, and prekeratin in frozen sections of normal human breast and infiltrating duct carcinomas by immunohistochemistry. A positive staining for gelsolin was observed in normal epithelial and myoepithelial cells but not in stromal fibroblasts. In contrast, no staining for gelsolin was detectable in carcinomatous cells, with the exception of remaining myoepithelial cells; myofibroblasts of the stromal reaction displayed an intense positive reaction. The absence of gelsolin staining in the epithelial cells of breast carcinoma may reflect their dedifferentiation or proliferative and invasive activities. The appearance of gelsolin in stromal cells raises the question as to what is its function in this situation. The immunohistochemical detection of gelsolin may be a useful adjunct to the study of mammary gland epithelium malignant transformation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong P. B., Lackie J. M. Studies of intercellular invasion in vitro using rabbit peritoneal neutrophil granulocytes (PMNS). I. Role of contact inhibition of locomotion. J Cell Biol. 1975 May;65(2):439–462. doi: 10.1083/jcb.65.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C., Borgia R., Rungger-Brändle E., Weil R., Gabbiani G. An actin-destabilizing factor is present in human plasma. Experientia. 1979 Aug 15;35(8):1039–1041. doi: 10.1007/BF01949928. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Janmey P. A., Yin H. L. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986 Oct;103(4):1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C., Patebex P., Gabbiani G. Human plasma actin-depolymerizing factor. Purification, biological activity and localization in leukocytes and platelets. Eur J Biochem. 1985 Jan 15;146(2):267–276. doi: 10.1111/j.1432-1033.1985.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Yin H. L., Stossel T. P. Reversibility of gelsolin/actin interaction in macrophages. Evidence of Ca2+-dependent and Ca2+-independent pathways. J Exp Med. 1987 Jan 1;165(1):97–106. doi: 10.1084/jem.165.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Csank-Brassert J., Schneeberger J. C., Kapanci Y., Trenchev P., Holborow E. J. Contractile proteins in human cancer cells. Immunofluorescent and electron microscopic study. Am J Pathol. 1976 Jun;83(3):457–474. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Heimark R. L., Schwartz S. M. Organization of actin cytoskeleton during early endothelial regeneration in vitro. J Cell Sci. 1984 Mar;66:39–50. doi: 10.1242/jcs.66.1.39. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Lombardi D., Schwartz S. M. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–2364. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. The cytoskeleton in cancer cells in animals and humans. Methods Achiev Exp Pathol. 1979;9:231–243. [PubMed] [Google Scholar]
- Gabbiani G., Trenchev P., Holborow E. J. Increase of contractile proteins in human cancer cells. Lancet. 1975 Oct 25;2(7939):796–797. doi: 10.1016/s0140-6736(75)80008-0. [DOI] [PubMed] [Google Scholar]
- Harmer J. H., Lolait S. J., Toh B. H., Pedersen J. S., Chaponnier C., Gabbiani G. Actin depolymerizing factor and the organization and distribution of actin in astrocytomas and meningiomas. Br J Cancer. 1983 Jul;48(1):89–93. doi: 10.1038/bjc.1983.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Schwartz J. H. Characterization of brevin, a serum protein that shortens actin filaments. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6798–6802. doi: 10.1073/pnas.78.11.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. E., Bamburg J. R., Weeds A. G. Actin filament disassembly in blood plasma. FEBS Lett. 1980 Nov 17;121(1):175–177. doi: 10.1016/0014-5793(80)81291-9. [DOI] [PubMed] [Google Scholar]
- Hinssen H., Small J. V., Sobieszek A. A Ca2+-dependent actin modulator from vertebrate smooth muscle. FEBS Lett. 1984 Jan 23;166(1):90–95. doi: 10.1016/0014-5793(84)80051-4. [DOI] [PubMed] [Google Scholar]
- Kanno K., Sasaki Y., Hidaka H. A Ca2+-sensitive actin regulatory protein from smooth muscle. FEBS Lett. 1985 May 20;184(2):202–206. doi: 10.1016/0014-5793(85)80607-4. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Kurth M. C., Wang L. L., Dingus J., Bryan J. Purification and characterization of a gelsolin-actin complex from human platelets. Evidence for Ca2+-insensitive functions. J Biol Chem. 1983 Sep 25;258(18):10895–10903. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Mehl R., Izumo S., Nadal-Ginard B., Yin H. L. Muscle is the major source of plasma gelsolin. J Biol Chem. 1988 Jun 15;263(17):8239–8243. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennette D. A. An improved mounting medium for immunofluorescence microscopy. Am J Clin Pathol. 1978 Jun;69(6):647–648. doi: 10.1093/ajcp/69.6.647. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Janmey P. A., Chaponnier C., Herbert T. J., Stossel T. P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987 Aug;105(2):833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low R. B., Chaponnier C., Gabbiani G. Organization of actin in epithelial cells during regenerative and neoplastic conditions. Correlation of morphologic, immunofluorescent, and biochemical findings. Lab Invest. 1981 Apr;44(4):359–367. [PubMed] [Google Scholar]
- Malech H. L., Lentz T. L. Microfilaments in epidermal cancer cells. J Cell Biol. 1974 Feb;60(2):473–482. doi: 10.1083/jcb.60.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructural comparison of the interface between epithelium and stroma in basal cell carcinoma and control human skin. Lab Invest. 1976 Aug;35(2):132–142. [PubMed] [Google Scholar]
- Nodes B. R., Shackelford J. E., Lebherz H. G. Synthesis and secretion of serum gelsolin by smooth muscle tissue. J Biol Chem. 1987 Apr 15;262(11):5422–5427. [PubMed] [Google Scholar]
- Norberg R., Thorstensson R., Utter G., Fagraeus A. F-Actin-depolymerizing activity of human serum. Eur J Biochem. 1979 Oct 15;100(2):575–583. doi: 10.1111/j.1432-1033.1979.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Skalli O., Jackson B., Schürch W., Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988 May 15;41(5):707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- Saurat J. H., Didierjean L., Skalli O., Siegenthaler G., Gabbiani G. The intermediate filament proteins of rabbit normal epidermal Merkel cells are cytokeratins. J Invest Dermatol. 1984 Dec;83(6):431–435. doi: 10.1111/1523-1747.ep12273528. [DOI] [PubMed] [Google Scholar]
- Schenk P. Microfilaments in human epithelial cancer cells. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975 Nov 25;84(3):241–256. doi: 10.1007/BF00312246. [DOI] [PubMed] [Google Scholar]
- Schürch W., Seemayer T. A., Lagacé R. Stromal myofibroblasts in primary invasive and metastatic carcinomas. A combined immunological, light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1981;391(2):125–139. doi: 10.1007/BF00437591. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Thelmo W. L. The myofibroblast: biologic, pathologic, and theoretical considerations. Pathol Annu. 1980;15(Pt 1):443–470. [PubMed] [Google Scholar]
- Seemayer T. A., Tremblay G., Shibata H. The unique association of mammary stromal sarcoma with intraductal carcinoma. Cancer. 1975 Aug;36(2):599–605. doi: 10.1002/1097-0142(197508)36:2<599::aid-cncr2820360242>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Vandekerckhove J., Gabbiani G. Actin-isoform pattern as a marker of normal or pathological smooth-muscle and fibroblastic tissues. Differentiation. 1987;33(3):232–238. doi: 10.1111/j.1432-0436.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Soua Z., Porte F., Harricane M. C., Feinberg J., Capony J. P. Bovine serum brevin. Purification by hydrophobic chromatography and properties. Eur J Biochem. 1985 Dec 2;153(2):275–287. doi: 10.1111/j.1432-1033.1985.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Sträuli P., Weiss L. Cell locomation and tumor penetration. Report on a workshop of the EORTC cell surface project group. Eur J Cancer. 1977 Jan;13(1):1–12. doi: 10.1016/0014-2964(77)90222-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Stromal aspects of breast carcinoma. Exp Mol Pathol. 1979 Aug;31(1):248–260. doi: 10.1016/0014-4800(79)90026-1. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Iida K., Janmey P. A. Identification of a polyphosphoinositide-modulated domain in gelsolin which binds to the sides of actin filaments. J Cell Biol. 1988 Mar;106(3):805–812. doi: 10.1083/jcb.106.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Kwiatkowski D. J., Mole J. E., Cole F. S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J Biol Chem. 1984 Apr 25;259(8):5271–5276. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]