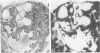Abstract
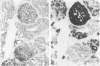
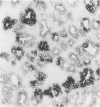
The clonal origin of thyroid tumors in female mice heterozygous for a deficiency of the X-linked enzyme glucose-6-phosphate dehydrogenase (G6PD) was studied. Tumor phenotype was demonstrated by enzyme histochemistry. Because monophenotypia is not synonymous with monoclonality, a method to estimate the degree of mingling of the two cellular phenotypes in normal tissue was devised. Twenty-five point three percent of 624 randomly chosen pairs of adjacent follicular cells were of unlike phenotype, suggesting that if tumors were derived from 2 or more cells at least a quarter would express polyphenotypia. Four hundred fifty-three thyroid lesions induced in 20 GPDX (enzyme-deficient) mice, 20 C3H (normal) mice, and 48 heterozygous (C3HxGPDX) mice by radiation and long-term goitrogen treatment were studied. One hundred twenty-eight adenomas (sharply defined or encapsulated hypercellular lesions) were found in heterozygotes; 108 (84%) were monophenotypic, and 20 (16%) were largely monophenotypic with degenerate areas or included normal cells. None were clearly polyphenotypic. Seventy-five nodules (circumscribed but not encapsulated, largely normocellular lesion with prominent stroma) were found in heterozygotes; 25 (33%) only were monophenotypic. It is concluded that thyroid adenomas are monoclonal and nodules polyclonal. The variegated pattern of polyphenotypia in the nodules together with their prominent stromal component leads to the suggestion that there is a causative role for the stroma in their generation.
Full text
PDF


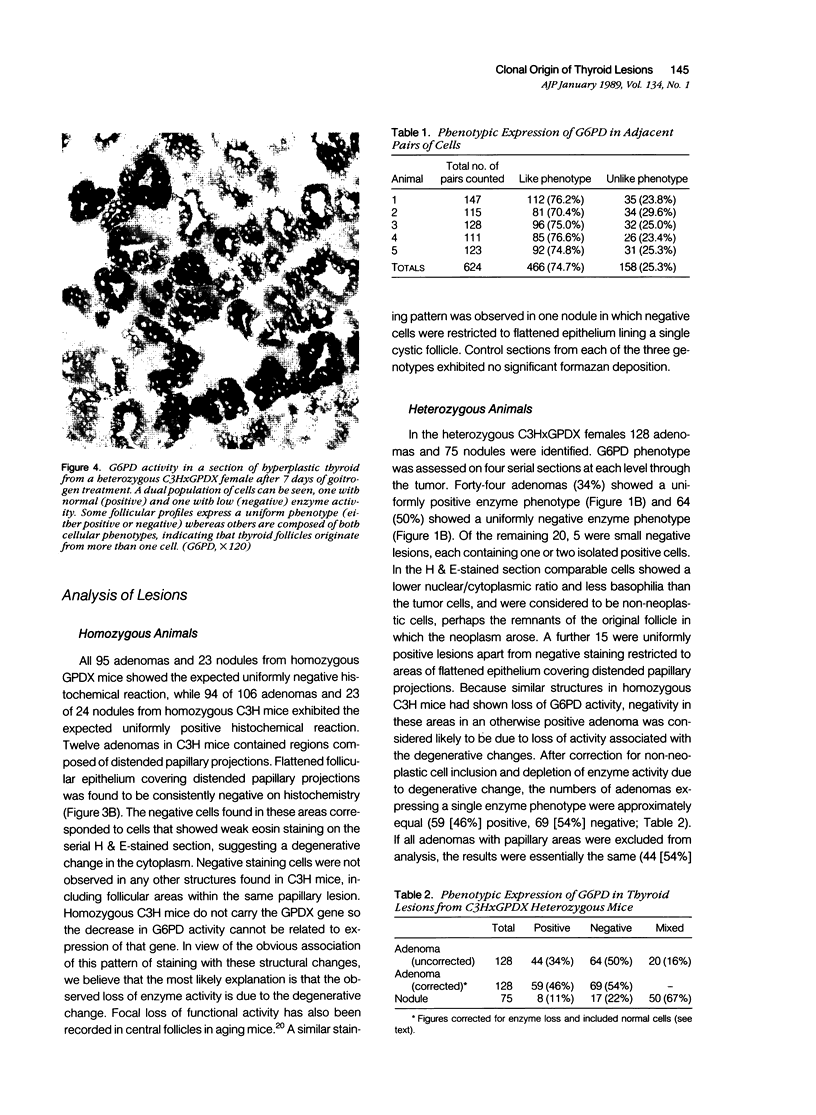


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Do cancers arise from a single transformed cell or is monoclonality of tumours a late event in carcinogenesis? Br J Cancer. 1985 Apr;51(4):453–457. doi: 10.1038/bjc.1985.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexhage H. A., Hammond L. J., Bitensky L., Chayen J., Bottazzo G. F., Doniach D. The involvement of the pentose shunt in thyroid metabolism after stimulation with TSH or with immunoglobulins from patients with thyroid disease. 1. The generation of NADPH in relation to stimulation of thyroid growth. Clin Endocrinol (Oxf) 1982 Jan;16(1):49–56. doi: 10.1111/j.1365-2265.1982.tb03146.x. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Jackson C. E., Block M. A., Greenawald K. A. Multicellular origin of parathyroid "adenomas". N Engl J Med. 1977 Sep 29;297(13):696–698. doi: 10.1056/NEJM197709292971304. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Sagebiel R. W., Gartler S. M., Rimoin D. L. Multiple cell origin of hereditary neurofibromas. N Engl J Med. 1971 Feb 11;284(6):298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- Frith C. H., Heath J. E. Morphological classification and incidence of thyroid tumors in untreated aged mice. J Gerontol. 1984 Jan;39(1):7–10. doi: 10.1093/geronj/39.1.7. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Ziprkowski L., Krakowski A., Ezra R., Szeinberg A., Adam A. Glucose-6-phosphate dehydrogenase mosaicism as a tracer in the study of hereditary multiple trichoepithelioma. Am J Hum Genet. 1966 May;18(3):282–287. [PMC free article] [PubMed] [Google Scholar]
- Gerber H., Peter H. J., Studer H. Age-related failure of endocytosis may be the pathogenetic mechanism responsible for "cold" follicle formation in the aging mouse thyroid. Endocrinology. 1987 May;120(5):1758–1764. doi: 10.1210/endo-120-5-1758. [DOI] [PubMed] [Google Scholar]
- Howell S., Wareham K. A., Williams E. D. Clonal origin of mouse liver cell tumors. Am J Pathol. 1985 Dec;121(3):426–432. [PMC free article] [PubMed] [Google Scholar]
- Iannaccone P. M., Gardner R. L., Harris H. The cellular origin of chemically induced tumours. J Cell Sci. 1978 Feb;29:249–269. doi: 10.1242/jcs.29.1.249. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Weinberg W. C., Berkwits L. A probabilistic model of mosaicism based on the histological analysis of chimaeric rat liver. Development. 1987 Feb;99(2):187–196. doi: 10.1242/dev.99.2.187. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Weinberg W. C., Deamant F. D. On the clonal origin of tumors: a review of experimental models. Int J Cancer. 1987 Jun 15;39(6):778–784. doi: 10.1002/ijc.2910390621. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- MINTZ B. GENETIC MOSAICISM IN ADULT MICE OF QUADRIPARENTAL LINEAGE. Science. 1965 May 28;148(3674):1232–1233. doi: 10.1126/science.148.3674.1232. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N. Chimeras vs X inactivation mosaics: significance of differences in pigment distribution. Dev Biol. 1974 May;38(1):202–207. doi: 10.1016/0012-1606(74)90272-3. [DOI] [PubMed] [Google Scholar]
- Peter H. J., Gerber H., Studer H., Smeds S. Pathogenesis of heterogeneity in human multinodular goiter. A study on growth and function of thyroid tissue transplanted onto nude mice. J Clin Invest. 1985 Nov;76(5):1992–2002. doi: 10.1172/JCI112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H. J., Studer H., Forster R., Gerber H. The pathogenesis of "hot" and "cold" follicles in multinodular goiters. J Clin Endocrinol Metab. 1982 Nov;55(5):941–946. doi: 10.1210/jcem-55-5-941. [DOI] [PubMed] [Google Scholar]
- Pretsch W., Charles D. J., Merkle S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet. 1988 Feb;26(1-2):89–103. doi: 10.1007/BF00555491. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Bücher T., Hartmann A., Linke I., Dünnwald M. Clonal growth of carcinogen-induced enzyme-deficient preneoplastic cell populations in mouse liver. Cancer Res. 1982 Aug;42(8):3220–3227. [PubMed] [Google Scholar]
- Reddy A. L., Fialkow P. J. Multicellular origin of fibrosarcomas in mice induced by the chemical carcinogen 3-methylcholanthrene. J Exp Med. 1979 Oct 1;150(4):878–887. doi: 10.1084/jem.150.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. L., Fialkow P. J. Papillomas induced by initiation-promotion differ from those induced by carcinogen alone. Nature. 1983 Jul 7;304(5921):69–71. doi: 10.1038/304069a0. [DOI] [PubMed] [Google Scholar]
- SANTLER J. E. Growth in the cell populations of the thyroid gland of rats treated with thiouracil. J Endocrinol. 1957 Jun;15(2):151–161. doi: 10.1677/joe.0.0150151. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Blount M. A., Ponder B. A. Immunochemical demonstration of the clonal organization of chimaeric mouse epidermis. Development. 1987 Jul;100(3):535–541. doi: 10.1242/dev.100.3.535. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Williams D., Williams E. D. The demonstration of tissue clonality by X-linked enzyme histochemistry. J Pathol. 1988 Jun;155(2):101–108. doi: 10.1002/path.1711550205. [DOI] [PubMed] [Google Scholar]
- Williams E. D., Wareham K. A., Howell S. Direct evidence for the single cell origin of mouse liver cell tumours. Br J Cancer. 1983 May;47(5):723–726. doi: 10.1038/bjc.1983.112. [DOI] [PMC free article] [PubMed] [Google Scholar]