Abstract
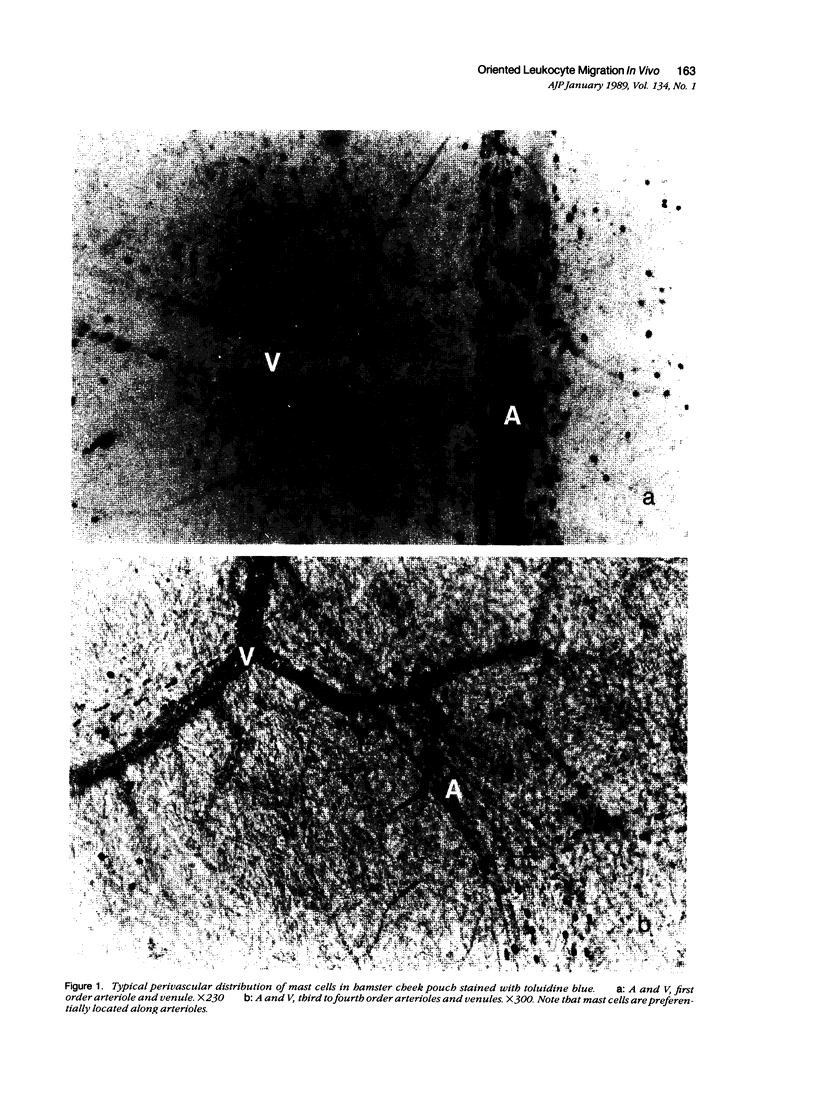
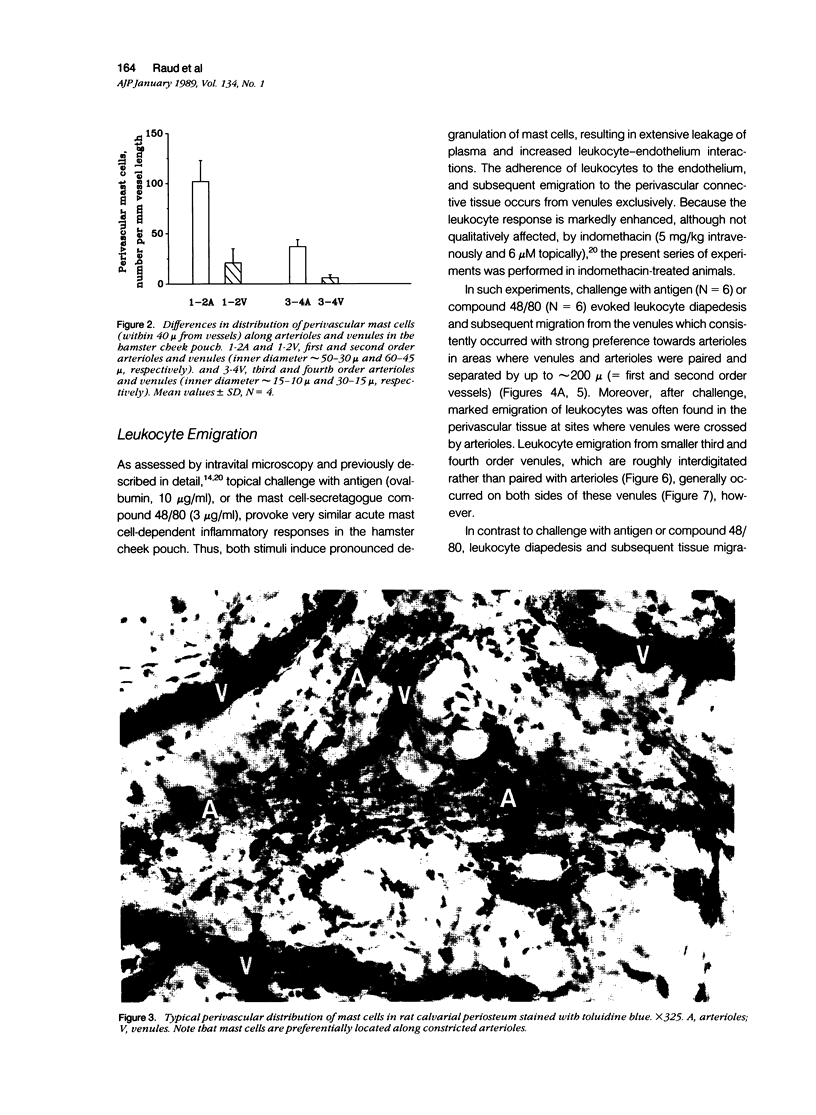
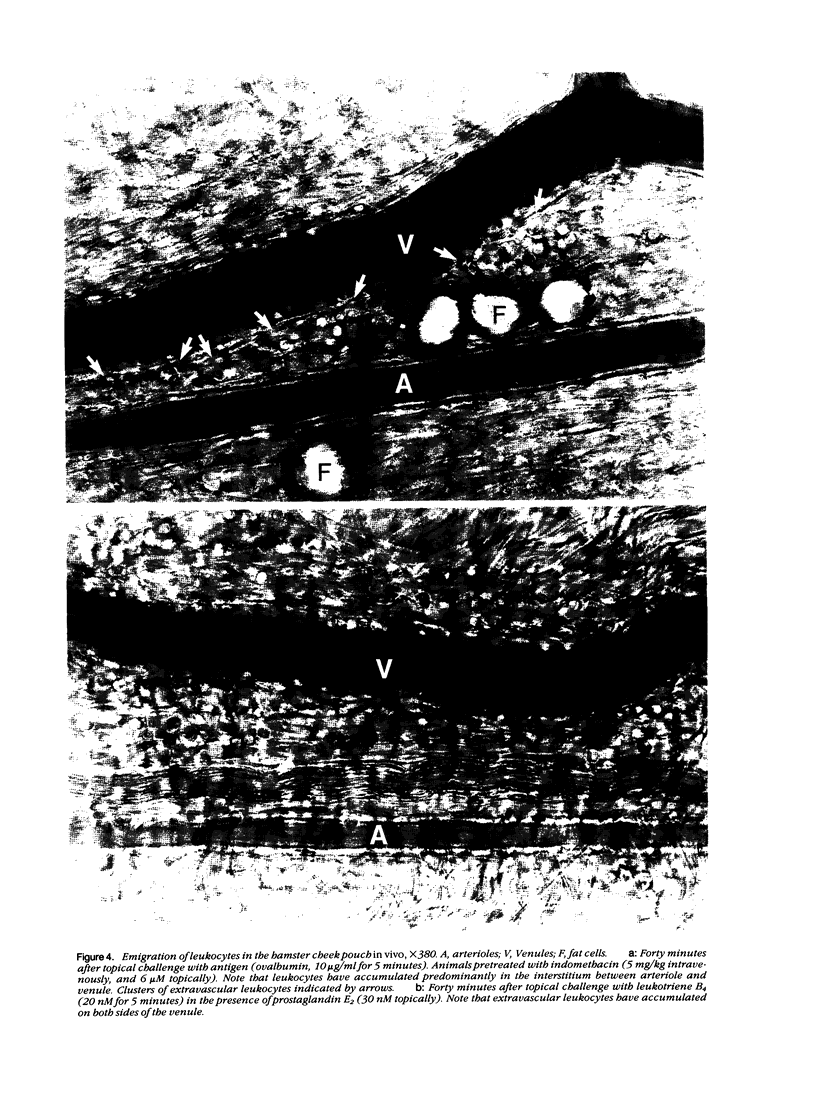
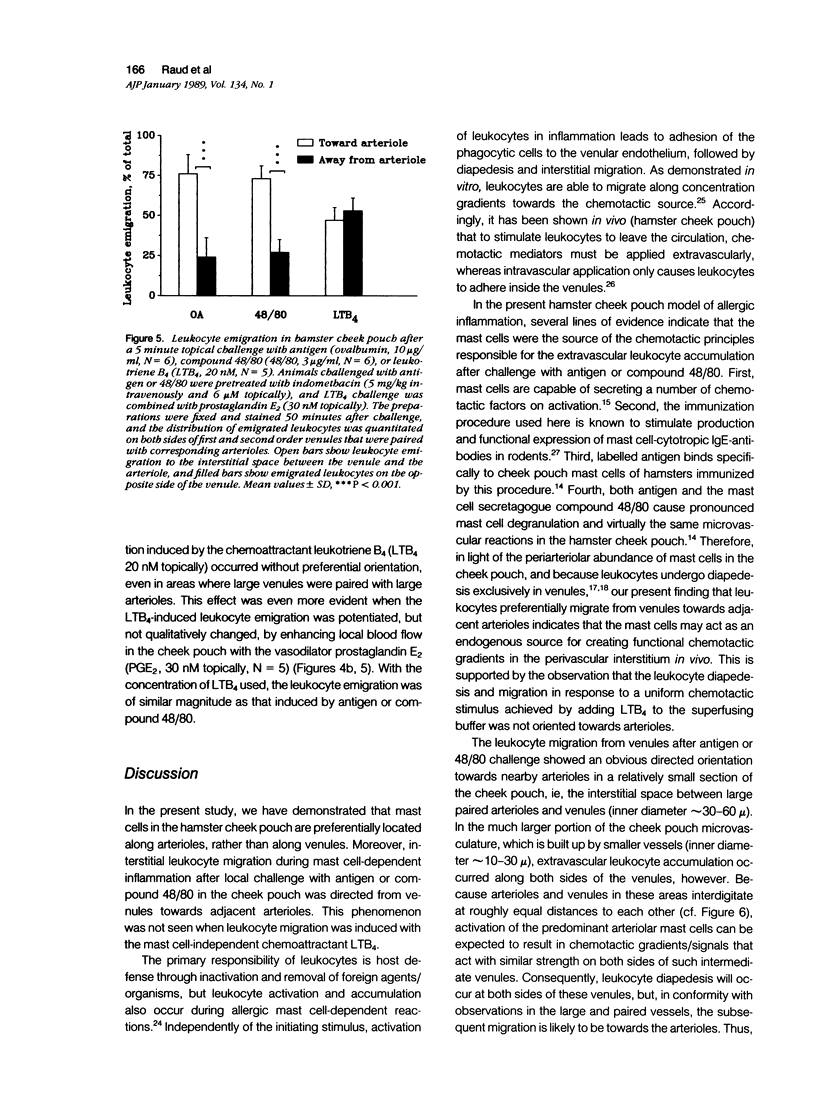
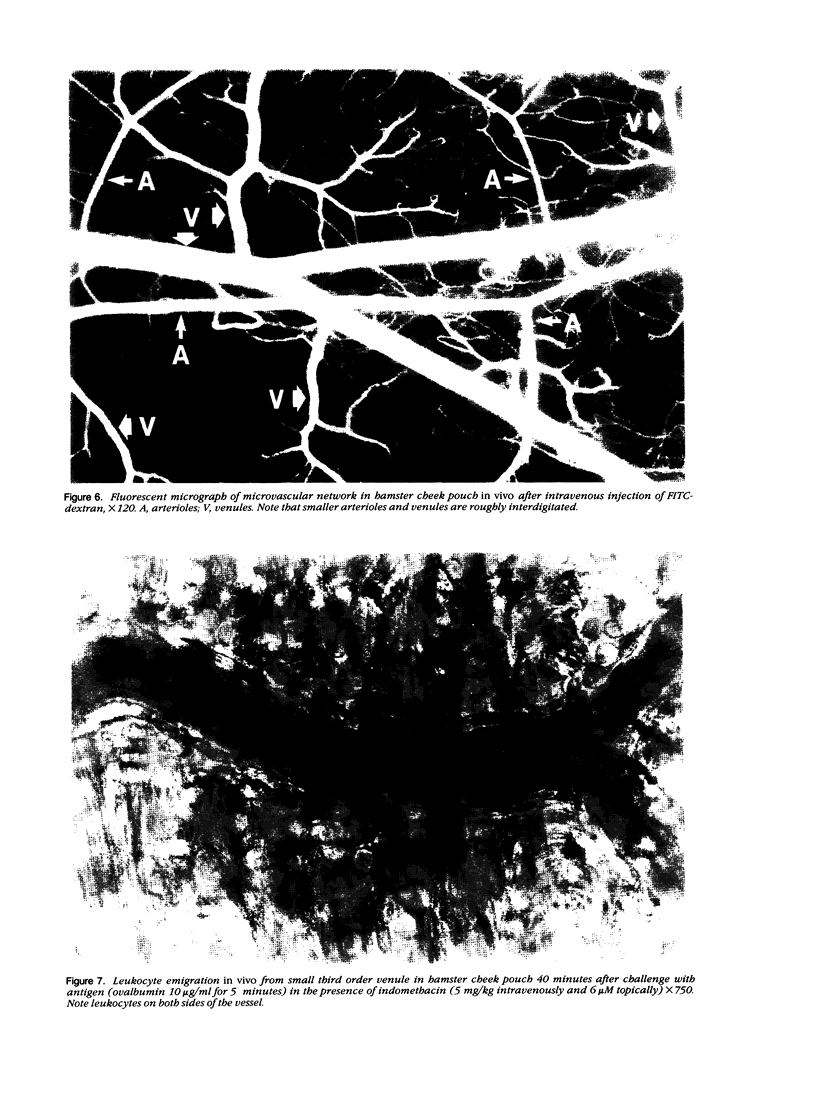
As studied by intravital microscopy, mast cell-dependent inflammatory reactions evoked by antigen or compound 48/80 in the hamster cheek pouch involved leakage of plasma and emigration of leukocytes exclusively from the venules. The leukocyte diapedesis and subsequent tissue migration induced by antigen or compound 48/80 were oriented from the venules towards adjacent arterioles. In contrast, leukocyte emigration induced by a mast cell-independent stimulus, leukotriene B4, did not show preferential orientation towards arterioles. Moreover, mast cells were abundant in the hamster cheek pouch, and they were localized predominantly along arterioles, rather than along venules. Because mast cells are considered to be the source of the chemotactic mediators causing the leukocyte emigration, the periarteriolar mast cell localization may be of functional significance by creating chemotactic gradients between arterioles and venules, thereby promoting oriented and effective interstitial migration of leukocytes. Whether or not a similar mechanism is operative in other species and tissues remains to be established, however, arteriolar predominance of mast cells was observed also in rat calvarial periosteum and in mouse skin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. K. Mediators of anaphylaxis and inflammation. Annu Rev Microbiol. 1982;36:371–413. doi: 10.1146/annurev.mi.36.100182.002103. [DOI] [PubMed] [Google Scholar]
- Bamberger U., Scheuber P. H., Sailer-Kramer B., Hammer D. K. Anti-idiotypic antibodies as staphylococcal enterotoxin receptor probes on monkey mast cells. Int Arch Allergy Appl Immunol. 1987;82(3-4):272–274. doi: 10.1159/000234204. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. J., Ferrer P. N., Gore R. W. Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am J Physiol. 1986 Feb;250(2 Pt 2):H291–H303. doi: 10.1152/ajpheart.1986.250.2.H291. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Piotrowski W. Peptides and histamine release. J Allergy Clin Immunol. 1984 Aug;74(2):127–131. doi: 10.1016/0091-6749(84)90274-4. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Basciano L. K., Siraganian R. P. Mouse mast cell activation and desensitization for immune aggregate-induced histamine release. J Immunol. 1982 Jul;129(1):314–319. [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Ghanem N. S., Assem E. S., Leung K. B., Pearce F. L. Guinea pig mast cells: comparative study on morphology, fixation and staining properties. Int Arch Allergy Appl Immunol. 1988;85(3):351–357. doi: 10.1159/000234531. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Triggering of histamine release from rat mast cells by divalent antibodies against IgE-receptors. J Immunol. 1978 Mar;120(3):800–805. [PubMed] [Google Scholar]
- Johnson A. R., Hugli T. E., Müller-Eberhard H. J. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975 Jun;28(6):1067–1080. [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. F., Tucker A., Munroe M. L., Reeves J. T. Lung mast cells and hypoxic pulmonary vasoconstriction in cats. Respiration. 1978;35(2):73–77. doi: 10.1159/000193862. [DOI] [PubMed] [Google Scholar]
- Mendonca V. O., Vugman I., Jamur M. C. Maturation of adult rat peritoneal and mesenteric mast cells. A morphological and histofluorescence study. Cell Tissue Res. 1986;243(3):635–639. doi: 10.1007/BF00218072. [DOI] [PubMed] [Google Scholar]
- Migally N. B., Tucker A., Greenlees K., Wright M., Zambernard J. Density and ultrastructure of mast cells in lung vessels of aging rats exposed to and recovering from chronic hypoxia. Cell Tissue Res. 1983;232(3):601–608. doi: 10.1007/BF00216432. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Kuwabara T., Chan C. C., Nussenblatt R. B., Metcalfe D. D., Gery I. An association between susceptibility to experimental autoimmune uveitis and choroidal mast cell numbers. J Immunol. 1984 Oct;133(4):1699–1701. [PubMed] [Google Scholar]
- Nowak J. Z., Nawrocki J., Maslinski C. Distribution and localization of histamine in bovine and rabbit eye. Agents Actions. 1984 Apr;14(3-4):335–340. doi: 10.1007/BF01973822. [DOI] [PubMed] [Google Scholar]
- Raud J., Dahlén S. E., Sydbom A., Lindbom L., Hedqvist P. Enhancement of acute allergic inflammation by indomethacin is reversed by prostaglandin E2: apparent correlation with in vivo modulation of mediator release. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2315–2319. doi: 10.1073/pnas.85.7.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELYE H., GABBIANI G., NIELSEN K. The "periosteal spread" technic for study of Mast cells. Proc Soc Exp Biol Med. 1963 Feb;112:460–463. doi: 10.3181/00379727-112-28077. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T. Regulation of reaginic antibody formation in animals. Prog Allergy. 1975;19:122–194. [PubMed] [Google Scholar]
- Tannenbaum S., Oertel H., Henderson W., Kaliner M. The biologic activity of mast cell granules. I. Elicitation of inflammatory responses in rat skin. J Immunol. 1980 Jul;125(1):325–335. [PubMed] [Google Scholar]
- Travis W. D., Li C. Y., Su W. P. Adult-onset urticaria pigmentosa and systemic mast cell disease. Am J Clin Pathol. 1985 Dec;84(6):710–714. doi: 10.1093/ajcp/84.6.710. [DOI] [PubMed] [Google Scholar]
- WEGELIUS O., HJELMMAN G. Vital staining of mast cells and fibrocytes. Acta Pathol Microbiol Scand. 1955;36(4):304–308. doi: 10.1111/j.1699-0463.1955.tb04619.x. [DOI] [PubMed] [Google Scholar]
- White M. V., Kaliner M. A. Neutrophils and mast cells. I. Human neutrophil-derived histamine-releasing activity. J Immunol. 1987 Sep 1;139(5):1624–1630. [PubMed] [Google Scholar]
- Williams A., Heath D., Harris P., Williams D., Smith P. Pulmonary mast cells in cattle and ilamas at high altitude. J Pathol. 1981 May;134(1):1–6. doi: 10.1002/path.1711340102. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H. Mechanisms of sensing chemical gradients by polymorphonuclear leukocytes. Nature. 1974 May 31;249(456):450–452. doi: 10.1038/249450a0. [DOI] [PubMed] [Google Scholar]
- de Kozak Y., Sainte-Laudy J., Benveniste J., Faure J. P. Evidence for immediate hypersensitivity phenomena in experimental autoimmune uveoretinitis. Eur J Immunol. 1981 Aug;11(8):612–617. doi: 10.1002/eji.1830110805. [DOI] [PubMed] [Google Scholar]