Abstract
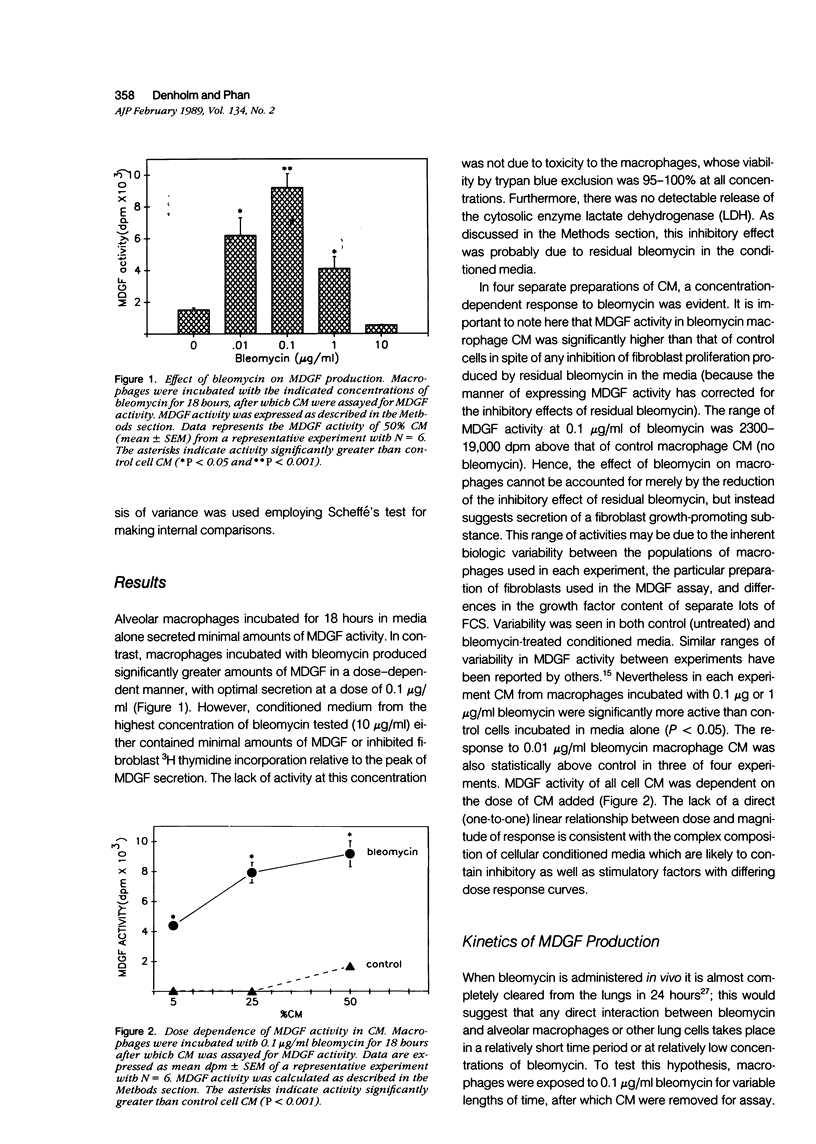
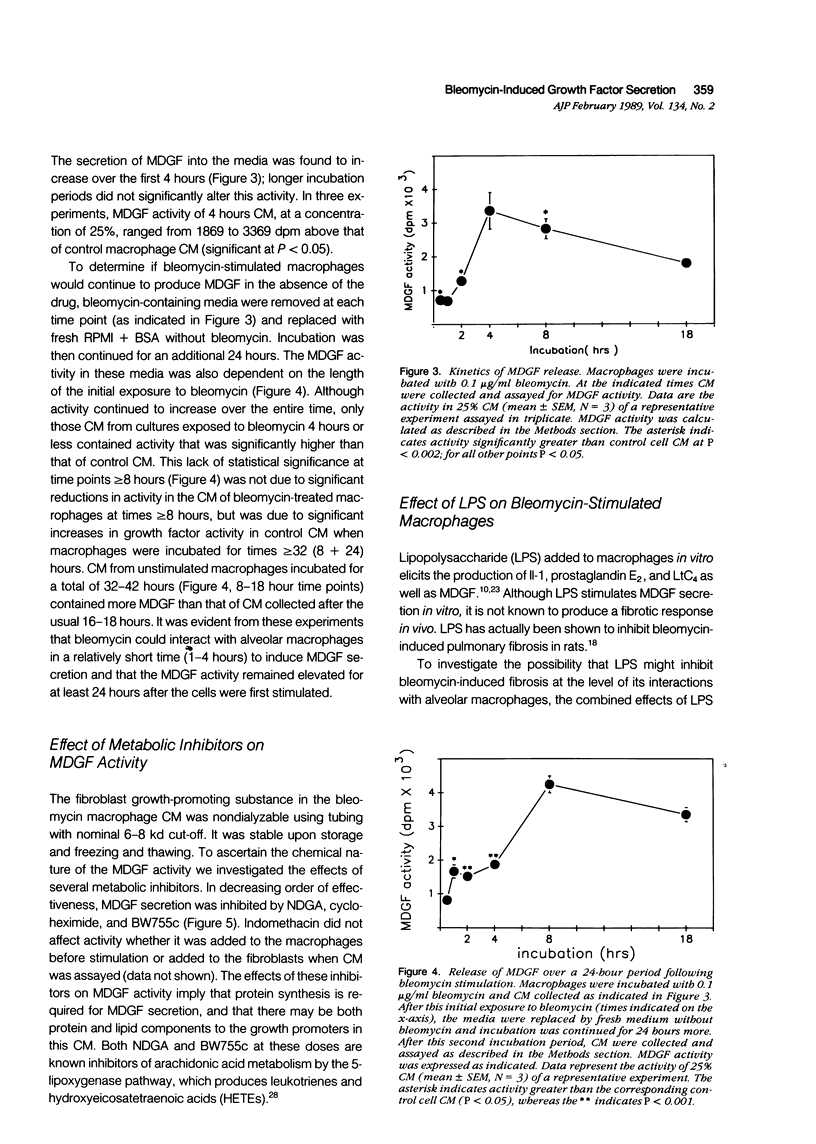
Previous work in this laboratory has demonstrated increased secretion of fibroblast growth factor (MDGF) activity by alveolar macrophages obtained from mice with bleomycin-induced pulmonary fibrosis. The mechanism by which bleomycin promotes this increase in MDGF secretion is not clear, however. The purpose of this study was to determine the direct effects of bleomycin on alveolar macrophages. Normal rat alveolar macrophages obtained by lavage were cultured in the presence or absence of bleomycin; conditioned media from these cultures were dialyzed to remove bleomycin and then assayed in vitro for MDGF activity. Alveolar macrophages incubated with 0.01 microgram to 1 microgram/ml bleomycin for 18 hours secreted significantly more MDGF than macrophages incubated without bleomycin. Viability of macrophages as determined by exclusion of trypan blue and release of LDH was unaffected by any dose tested. Maximal MDGF production was seen with bleomycin doses of greater than or equal to 0.1 microgram/ml. When alveolar macrophages were incubated with 0.1 microgram/ml bleomycin for 0.5-18 hours, MDGF activity was detected as early as 1 hour, with peak responses found at 4-8 hours. Macrophages stimulated with bleomycin continued to produce significant amounts of MDGF even after bleomycin was removed and replaced with fresh (bleomycin-free) media. MDGF secretion by bleomycin-stimulated alveolar macrophages was inhibited by cycloheximide, and the 5-lipoxygenase inhibitors NDGA (nordihydroguairetic acid) and BW755c, indicating not only a requirement for protein synthesis but also for metabolites of the 5-lipoxygenase pathway of arachidonic acid metabolism for full expression of activity(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M., Heppleston A. G. Fibrogenesis by mineral fibres: an in-vitro study of the roles of the macrophage and fibre length. Br J Exp Pathol. 1984 Feb;65(1):91–99. [PMC free article] [PubMed] [Google Scholar]
- Aalto M., Potila M., Kulonen E. The effect of silica-treated macrophages on the synthesis of collagen and other proteins in vitro. Exp Cell Res. 1976 Jan;97:193–202. doi: 10.1016/0014-4827(76)90668-6. [DOI] [PubMed] [Google Scholar]
- Absher M., Hildebran J., Trombley L., Woodcock-Mitchell J., Marsh J. Characteristics of cultured lung fibroblasts from bleomycin-treated rats. Comparisons with in vitro exposed normal fibroblasts. Am Rev Respir Dis. 1984 Jan;129(1):125–129. doi: 10.1164/arrd.1984.129.1.125. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974 Nov;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Varesio L. Role of protein synthesis in the activation of cytotoxic mouse macrophages by lymphokines. Cell Immunol. 1984 Apr 15;85(1):15–24. doi: 10.1016/0008-8749(84)90273-9. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Bleomycin-induced pulmonary fibrosis in hamsters. An alveolar macrophage product increases fibroblast prostaglandin E2 and cyclic adenosine monophosphate and suppresses fibroblast proliferation and collagen production. J Clin Invest. 1983 Dec;72(6):2082–2091. doi: 10.1172/JCI111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Starcher B. C., Uitto J. Bleomycin-induced synthesis of type I procollagen by human lung and skin fibroblasts in culture. Biochim Biophys Acta. 1980 Aug 13;631(2):359–370. doi: 10.1016/0304-4165(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The macrophage--versatile element of inflammation. Harvey Lect. 1981 1982;77:63–80. [PubMed] [Google Scholar]
- Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med. 1978 Feb 16;298(7):353–357. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Sadowski S., Galavage M., Goldenberg M., Subers E., Kuehl F. A., Jr, Bonney R. J. Pharmacological effects of non-steroidal antiinflammatory agents on prostaglandin and leukotriene synthesis in mouse peritoneal macrophages. Biochem Pharmacol. 1983 Aug 1;32(15):2319–2322. doi: 10.1016/0006-2952(83)90179-x. [DOI] [PubMed] [Google Scholar]
- Jordana M., Richards C., Irving L. B., Gauldie J. Spontaneous in vitro release of alveolar-macrophage cytokines after the intratracheal instillation of bleomycin in rats. Characterization and kinetic studies. Am Rev Respir Dis. 1988 May;137(5):1135–1140. doi: 10.1164/ajrccm/137.5.1135. [DOI] [PubMed] [Google Scholar]
- Kouzan S., Nolan R. D., Fournier T., Bignon J., Eling T. E., Brody A. R. Stimulation of arachidonic acid metabolism by adherence of alveolar macrophages to a plastic substrate. Modulation by fetal bovine serum. Am Rev Respir Dis. 1988 Jan;137(1):38–43. doi: 10.1164/ajrccm/137.1.38. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Secretion of macrophage-derived growth factor during acute lung injury induced by bleomycin. J Leukoc Biol. 1985 Jan;37(1):1–14. doi: 10.1002/jlb.37.1.1. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Mouton C., Higashi G. I. Role of lipoxygenase products in murine pulmonary granuloma formation. J Clin Invest. 1984 Aug;74(2):514–524. doi: 10.1172/JCI111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Lazo J. S., Pham E. T. Pulmonary fate of [3H]bleomycin A2 in mice. J Pharmacol Exp Ther. 1984 Jan;228(1):13–18. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Lemaire I., Beaudoin H., Massé S., Grondin C. Alveolar macrophage stimulation of lung fibroblast growth in asbestos-induced pulmonary fibrosis. Am J Pathol. 1986 Feb;122(2):205–211. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Majeau G. R., Unanue E. R., Cotran R. S. Stimulation of human monocyte/macrophage-derived growth factor (MDGF) production by plasma fibronectin. Am J Pathol. 1983 Jun;111(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Moseley P. L., Hemken C., Hunninghake G. W. Augmentation of fibroblast proliferation by bleomycin. J Clin Invest. 1986 Nov;78(5):1150–1154. doi: 10.1172/JCI112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka K., Murota S. I., Mori Y. Stimulatory effect of bleomycin on the synthesis of acidic glycosaminoglycans in cultured fibroblasts derived from rat carrageenin granuloma. Biochim Biophys Acta. 1976 Sep 24;444(2):359–368. doi: 10.1016/0304-4165(76)90379-2. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Fantone J. C. Inhibition of bleomycin-induced pulmonary fibrosis by lipopolysaccharide. Lab Invest. 1984 May;50(5):587–591. [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Inhibition of bleomycin-induced pulmonary fibrosis by nordihydroguaiaretic acid. The role of alveolar macrophage activation and mediator production. Am J Pathol. 1986 Aug;124(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., McGarry B. M., Loeffler K. M., Kunkel S. L. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988 Apr 19;27(8):2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- Phan S. H., McGarry B. M., Loeffler K. M., Kunkel S. L. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol. 1987 Aug;42(2):106–113. doi: 10.1002/jlb.42.2.106. [DOI] [PubMed] [Google Scholar]
- Starcher B. C., Kuhn C., Overton J. E. Increased elastin and collagen content in the lungs of hamsters receiving an intratracheal injection of bleomycin. Am Rev Respir Dis. 1978 Feb;117(2):299–305. doi: 10.1164/arrd.1978.117.2.299. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]


