Abstract
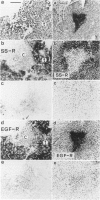
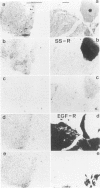
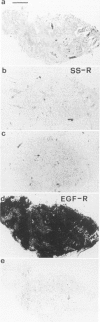
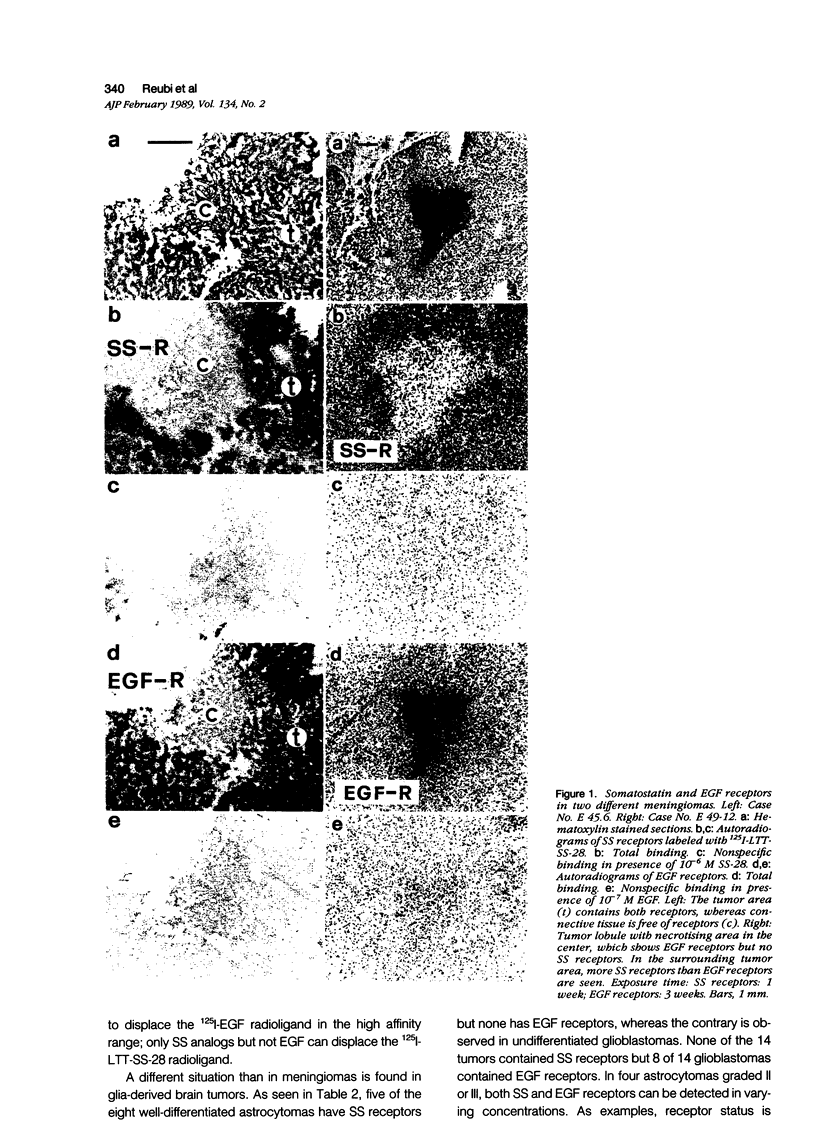
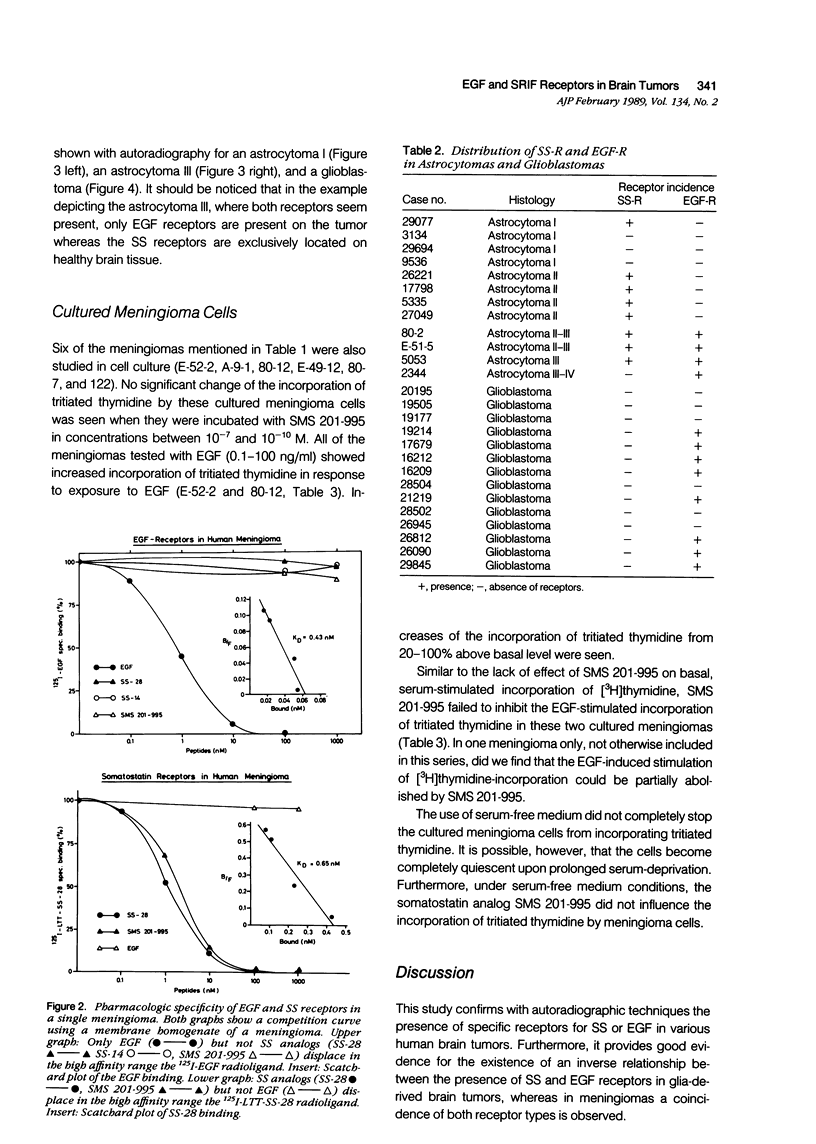
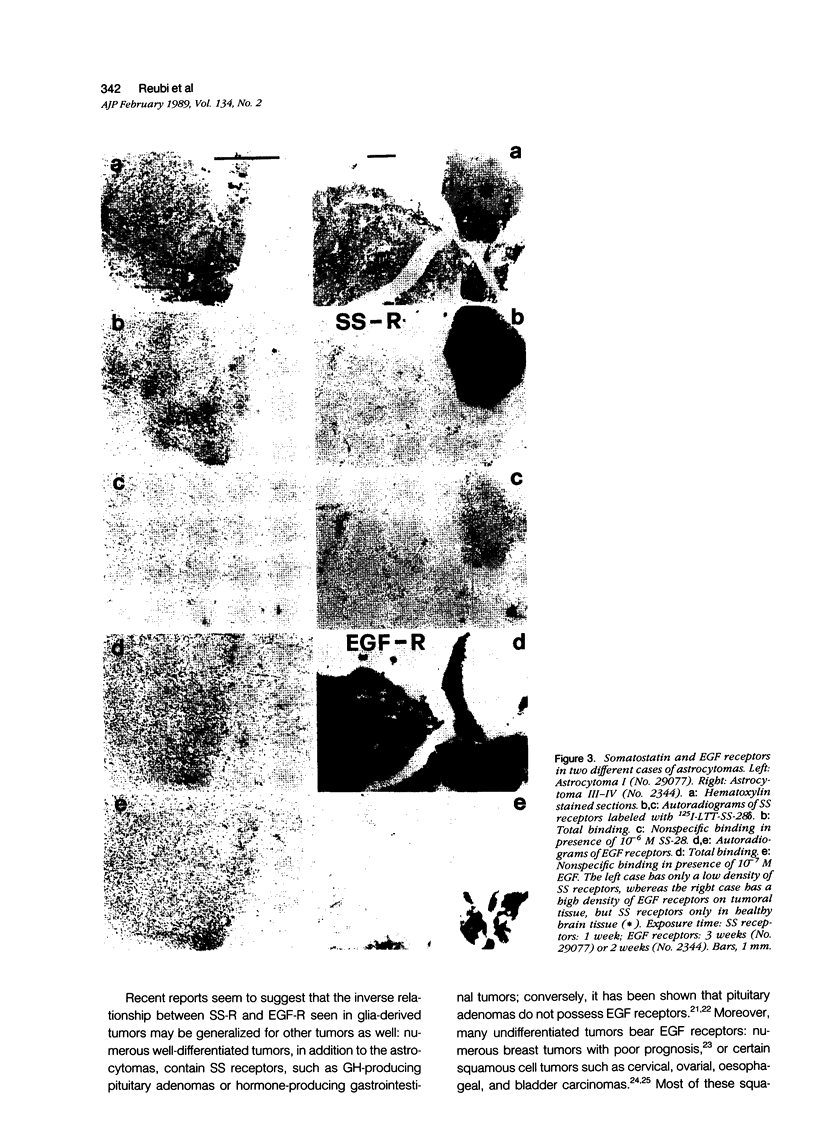
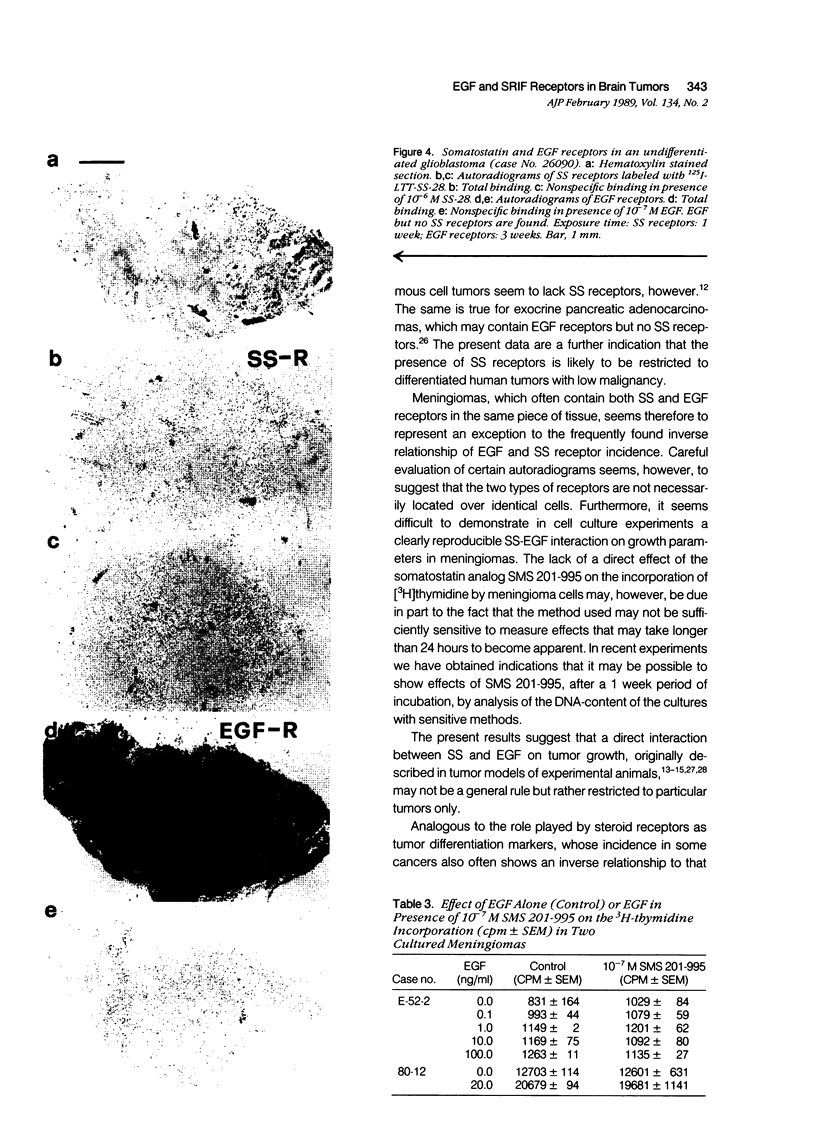
Somatostatin (SS) receptors as well as EGF receptors have been shown to be present in various brain tumors such as meningiomas or glia-derived tumors. Using receptor autoradiography for both receptors, their localization on adjacent tumor sections was investigated and a correlation was attempted. In glia-derived tumors, there was an inverse relationship for the incidence of the two receptors in individual tumors: in a majority of cases (five of eight) of well-differentiated astrocytomas (I-II), SS receptors were present, but in none of the cases (zero of eight) EGF receptors were detected. In undifferentiated glioblastomas, the reverse situation was observed, no SS receptors were found (0 of 14) but EGF receptors were present in a majority of tumors (8 of 14). In astrocytomas III both types of receptors were normally seen. These data suggest that in glia-derived tumors, SS receptors are markers for the well-differentiated cases as opposed to EGF receptors. In meningiomas, SS receptors are found in all (27 of 27) tumors and EGF receptors in a large percentage (23 of 27) of the same tumors. However, in some cases a coincidence of both receptors on the same cell can be excluded. Furthermore, no effect of the SS analog SMS 201-995 on basal or EGF-stimulated growth of meningiomas in culture could be detected. Nevertheless, the coexistence of the two receptor types in meningiomas may be suggestive for a potential functional interaction between EGF and SS.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks-Schlegel S. P., Quintero J. Human esophageal carcinoma cells have fewer, but higher affinity epidermal growth factor receptors. J Biol Chem. 1986 Apr 5;261(10):4359–4362. [PubMed] [Google Scholar]
- Birman P., Michard M., Li J. Y., Peillon F., Bression D. Epidermal growth factor-binding sites, present in normal human and rat pituitaries, are absent in human pituitary adenomas. J Clin Endocrinol Metab. 1987 Aug;65(2):275–281. doi: 10.1210/jcem-65-2-275. [DOI] [PubMed] [Google Scholar]
- Bonfils S. New somatostatin molecule for management of endocrine tumours. Gut. 1985 May;26(5):433–437. doi: 10.1136/gut.26.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conteas C. N., Majumdar A. P. The effects of gastrin, epidermal growth factor, and somatostatin on DNA synthesis in a small intestinal crypt cell line (IEC-6). Proc Soc Exp Biol Med. 1987 Mar;184(3):307–311. doi: 10.3181/00379727-184-42484. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27(1):63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Marsden J. J., Whittle N., Ward B., Bobrow L., Waterfield M. D. Expression of epidermal growth factor receptors on human cervical, ovarian, and vulval carcinomas. Cancer Res. 1986 Jan;46(1):285–292. [PubMed] [Google Scholar]
- Hierowski M. T., Liebow C., du Sapin K., Schally A. V. Stimulation by somatostatin of dephosphorylation of membrane proteins in pancreatic cancer MIA PaCa-2 cell line. FEBS Lett. 1985 Jan 7;179(2):252–256. doi: 10.1016/0014-5793(85)80529-9. [DOI] [PubMed] [Google Scholar]
- Kraenzlin M. E., Ch'ng J. L., Wood S. M., Carr D. H., Bloom S. R. Long-term treatment of a VIPoma with somatostatin analogue resulting in remission of symptoms and possible shrinkage of metastases. Gastroenterology. 1985 Jan;88(1 Pt 1):185–187. doi: 10.1016/s0016-5085(85)80153-0. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Koper J. W., Reubi J. C. Potential role of somatostatin analogues in the treatment of cancer. Eur J Clin Invest. 1987 Aug;17(4):281–287. doi: 10.1111/j.1365-2362.1987.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Uitterlinden P., Verschoor L., van Dongen K. J., del Pozo E. Long-term treatment of acromegaly with the somatostatin analogue SMS 201-995. N Engl J Med. 1985 Dec 19;313(25):1576–1580. doi: 10.1056/NEJM198512193132504. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Razon N., Bartal A. D., Yarden Y., Schlessinger J., Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984 Feb;44(2):753–760. [PubMed] [Google Scholar]
- Maddy S. Q., Chisholm G. D., Hawkins R. A., Habib F. K. Localization of epidermal growth factor receptors in the human prostate by biochemical and immunocytochemical methods. J Endocrinol. 1987 Apr;113(1):147–153. doi: 10.1677/joe.0.1130147. [DOI] [PubMed] [Google Scholar]
- Mascardo R. N., Sherline P. Somatostatin inhibits rapid centrosomal separation and cell proliferation induced by epidermal growth factor. Endocrinology. 1982 Oct;111(4):1394–1396. doi: 10.1210/endo-111-4-1394. [DOI] [PubMed] [Google Scholar]
- Moreau J. P., DeFeudis F. V. Pharmacological studies of somatostatin and somatostatin-analogues: therapeutic advances and perspectives. Life Sci. 1987 Feb 2;40(5):419–437. doi: 10.1016/0024-3205(87)90107-x. [DOI] [PubMed] [Google Scholar]
- Neal D. E., Marsh C., Bennett M. K., Abel P. D., Hall R. R., Sainsbury J. R., Harris A. L. Epidermal-growth-factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet. 1985 Feb 16;1(8425):366–368. doi: 10.1016/s0140-6736(85)91386-8. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Srikant C. B. Somatostatin mediation of adenohypophysial secretion. Annu Rev Physiol. 1986;48:551–567. doi: 10.1146/annurev.ph.48.030186.003003. [DOI] [PubMed] [Google Scholar]
- Presky D. H., Schonbrunn A. Receptor-bound somatostatin and epidermal growth factor are processed differently in GH4C1 rat pituitary cells. J Cell Biol. 1986 Mar;102(3):878–888. doi: 10.1083/jcb.102.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Cortès R., Maurer R., Probst A., Palacios J. M. Distribution of somatostatin receptors in the human brain: an autoradiographic study. Neuroscience. 1986 Jun;18(2):329–346. doi: 10.1016/0306-4522(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Heitz P. U., Gyr K. Vasoactive intestinal peptide producing tumour contains high density of somatostatin receptors. Lancet. 1987 Mar 28;1(8535):741–742. doi: 10.1016/s0140-6736(87)90375-8. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Heitz P. U., Landolt A. M. Visualization of somatostatin receptors and correlation with immunoreactive growth hormone and prolactin in human pituitary adenomas: evidence for different tumor subclasses. J Clin Endocrinol Metab. 1987 Jul;65(1):65–73. doi: 10.1210/jcem-65-1-65. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Horisberger U., Essed C. E., Jeekel J., Klijn J. G., Lamberts S. W. Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology. 1988 Sep;95(3):760–763. doi: 10.1016/s0016-5085(88)80025-8. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Häcki W. H., Lamberts S. W. Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors. J Clin Endocrinol Metab. 1987 Dec;65(6):1127–1134. doi: 10.1210/jcem-65-6-1127. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Lang W., Maurer R., Koper J. W., Lamberts S. W. Distribution and biochemical characterization of somatostatin receptors in tumors of the human central nervous system. Cancer Res. 1987 Nov 1;47(21):5758–5764. [PubMed] [Google Scholar]
- Reubi J. C., Maurer R., Klijn J. G., Stefanko S. Z., Foekens J. A., Blaauw G., Blankenstein M. A., Lamberts S. W. High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab. 1986 Aug;63(2):433–438. doi: 10.1210/jcem-63-2-433. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Maurer R., von Werder K., Torhorst J., Klijn J. G., Lamberts S. W. Somatostatin receptors in human endocrine tumors. Cancer Res. 1987 Jan 15;47(2):551–558. [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Sherbet G. V., Harris A. L. Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet. 1985 Feb 16;1(8425):364–366. doi: 10.1016/s0140-6736(85)91385-6. [DOI] [PubMed] [Google Scholar]